Instructions for use D-Vapor/D-Vapor 3000 91
Cleaning and disinfection
Reprocessing procedures
Classification of medical devices
For reprocessing, medical devices and their
components are classified according to the type of
application and the risk resulting from it:
– Non-critical medical devices: Surfaces
accessible to the user, such as device surfaces,
cables
– Semi-critical medical devices: Parts conducting
breathing gas, e.g., breathing hoses, masks
With regard to reprocessing, this medical device is
a non-critical medical device.
Testing of procedures and agents
The cleaning and disinfection of medical products
has been tested with the following procedures and
agents.
At the time of testing, the following agents exhibited
good material compatibility:
– Buraton 10F made by Schülke & Mayr (basic
active ingredient: aldehyde)
– Dismozon pur made by Bode Chemie (basic
active ingredient: oxygen-releasing agent)
Manual disinfection and simultaneous cleaning
Dräger recommends using a surface disinfectant
from the following list. Other disinfectants are used
at own risk.
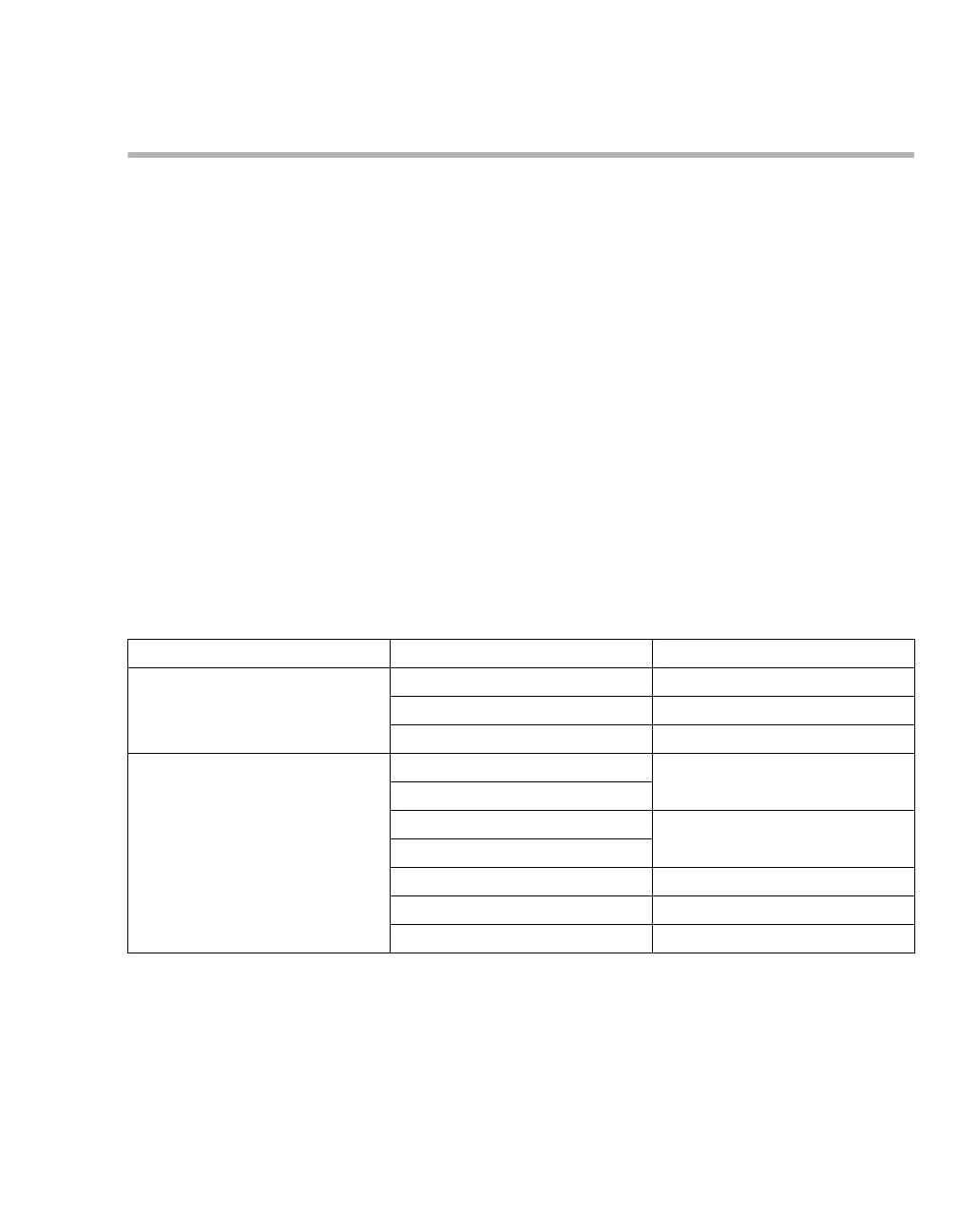
Class of active ingredient Product name Manufacturer
Chlorine-releasing agents Actichlor
®
plus Ecolab
Klorsept
®
17 Medentech
BruTab 6S
®
Brulin
Oxygen-releasing agents Descogen
®
Liquid Antiseptica
Descogen
®
Liquid r.f.u.
Dismozon
®
plus Bode Chemie
Dismozon
®
pur
Oxycide
®
Ecolab USA
Perform
®
Schülke & Mayr
Virkon
®
DuPont

 Loading...
Loading...