Revision D 250 Series Maternal/Fetal Monitor 4-19
2020551-001
Maintenance: Checkout
US2: Connect an US test body to the monitor’s front panel US2 input. The US
test body should be an ultrasound transducer wrapped in aluminum foil.
Measure the voltage breakdown.
F pass F fail
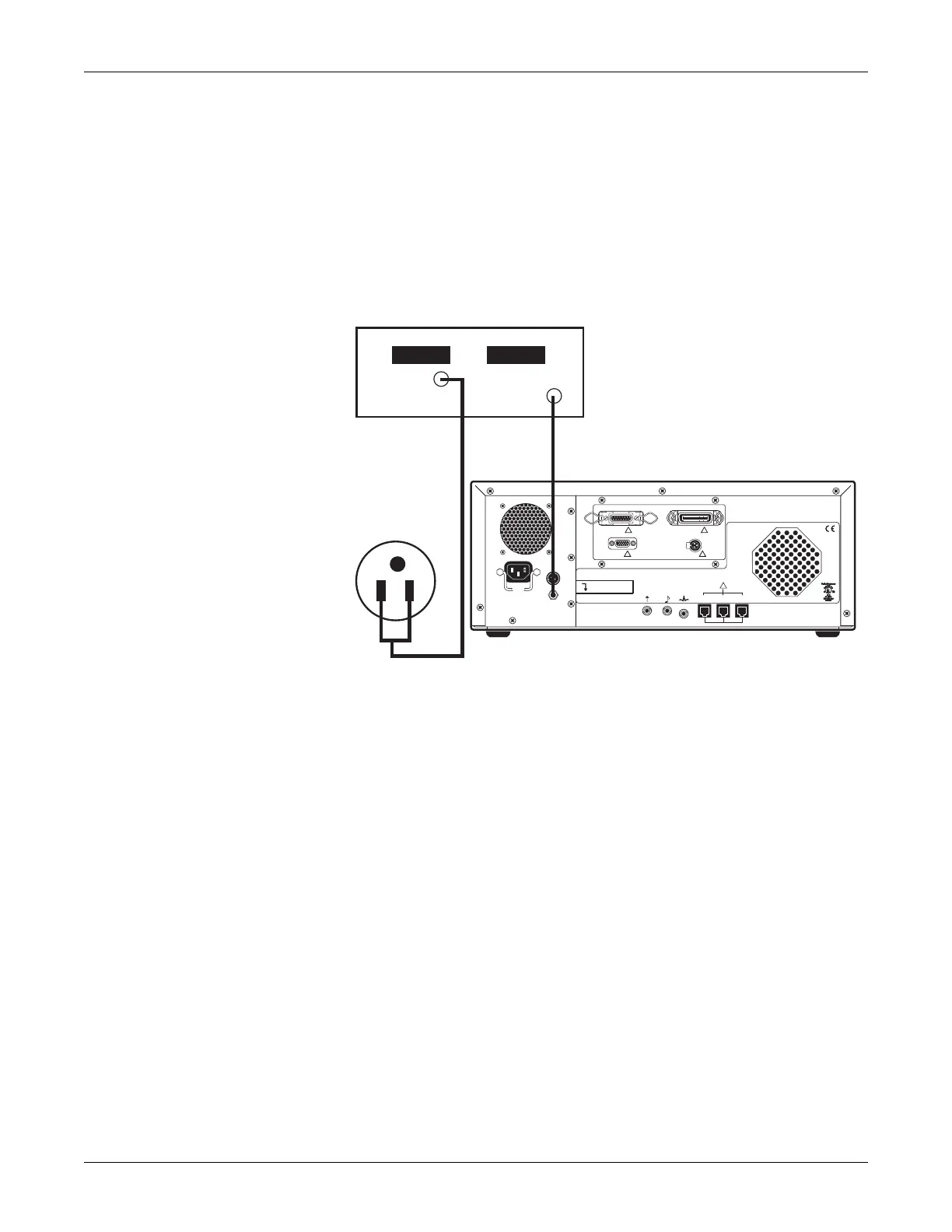
Mains–to–Chassis Using DC Voltage for 1 Minute
Connect the monitor to the hi-pot tester as shown in the following figure.
NOTE: The hi-pot tester voltage is 2.121 kVdc.
Mains-to-Chassis Using DC Voltage for 1 Minute
Checkout
General
Like all electronic monitoring devices, internal and external components are subject
to fatigue, wear, and the potential for failure over time and under varying conditions
of use. Additionally, events such as dropping the monitor, spilling liquids on the
monitor, or crimping the lead wires or patient cables can cause damage which may
affect the overall system performance. Therefore, between factory service visits it is
necessary that the proper operation of each monitor be verified by performing the
functional checkout procedure described in this section. This procedure should be
completed prior to initially placing the monitor on a patient, when monitor
performance needs to be verified, on a semi-annual basis, or more frequently as
dictated by your equipment maintenance and management policies.
120
24
0
CAUTION: FEDERAL
LAW RESTRICTS THIS
DEVICE TO SALE BY
OR ON THE ORDER OF
A PHYSICIAN.
J103
J102
J109
RS232C
J110
RS232C
J111
RS232C
!
J101
!
J104
PUSH
!
!
!
100 -120V ~ 50-60HZ 100W
Munzinger Strae 3-5; D-79111 Freiburg Germany
GE Medical Systems Information Technolgies GmbH
European Representative
8200 West Tower Avenue; Milwaukee, WI, USA
GE Medical Systems Information Technologies, Inc
0086
CANADIAN PATS. 1,057,360 1,214,143
U.S. PATS. 3,982,528 4,533,926 4,573,479
DC Out
Ground

 Loading...
Loading...