4 - 13
Transpector XPR 3+ Operating Manual
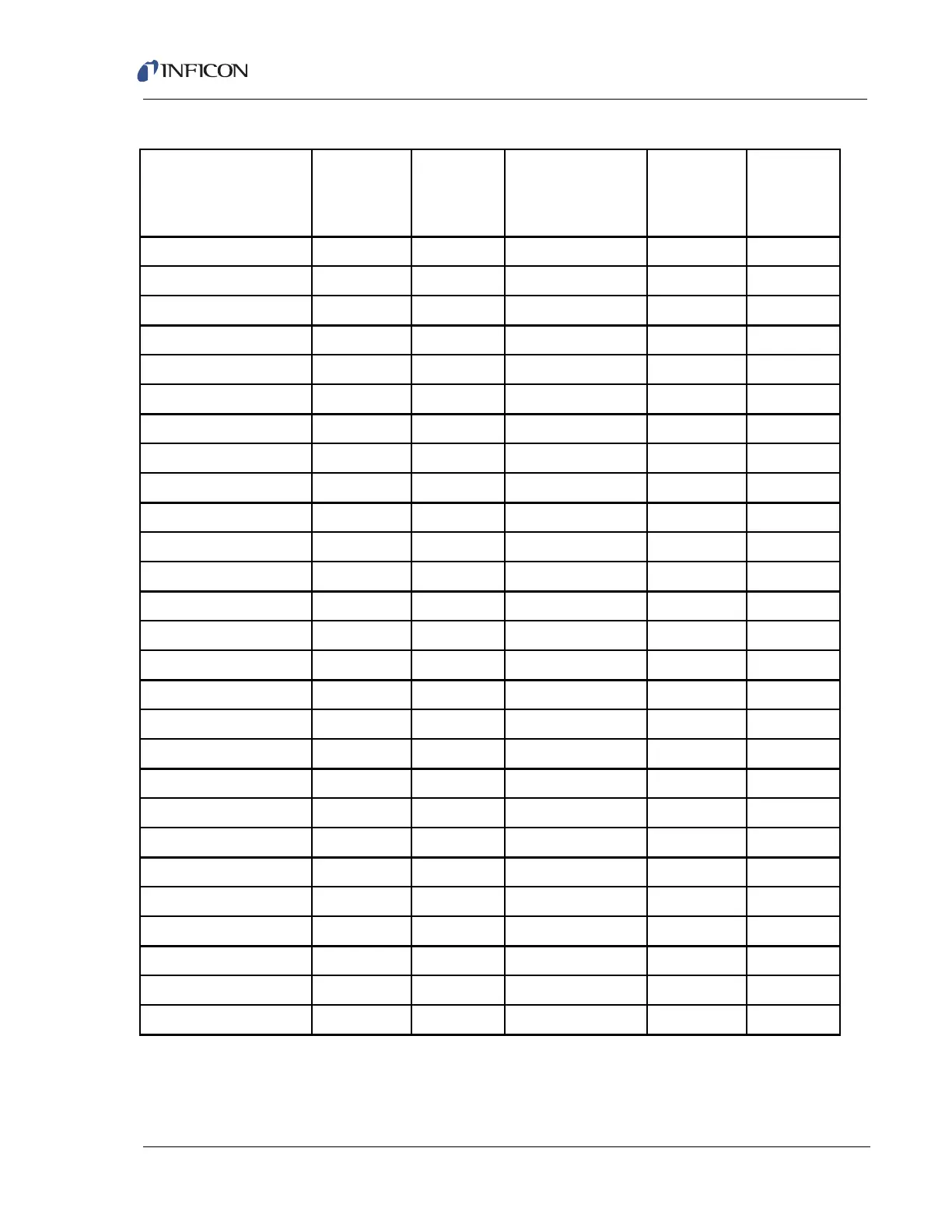
Table 4-5 Ionization probabilities for some common substances
Substance Formula
Relative
Ionization
Gauge
Sensitivity
Substance Formula
Relative
Ionization
Gauge
Sensitivity
Acetone (CH
3
)
2
CO 3.6 Hydrogen chloride HCl 1.6
Air 1.0 Hydrogen fluoride HF 1.4
Ammonia NH
3
1.3 Hydrogen iodide HI 3.1
Argon Ar 1.2 Hydrogen sulfide H
2
S2.2
Benzene C
6
H
6
5.9 Krypton Kr 1.7
Benzoic acid C
6
H
5
COOH 5.5 Lithium Li 1.9
Bromine Br
2
3.8 Methane CH
4
1.6
Butane C
4
H
10
4.9 Methanol CH
3
OH 1.8
Carbon dioxide CO
2
1.4 Neon Ne 0.23
Carbon disulfide CS
2
4.8 Nitrogen N
2
1.0
Carbon monoxide CO 1.05 Nitric oxide NO 1.2
Carbon tetrachloride CCl
4
6.0 Nitrous oxide N
2
O1.7
Chlorobenzene C
6
H
5
Cl 7.0 Oxygen O
2
1.0
Chloroethane C
2
H
5
Cl 4.0 n-Pentane C
5
H
12
6.0
Chloroform CHCl
3
4.8 Phenol C
6
H
5
OH 6.2
Chloromethane CH
3
Cl 3.1 Phosphine PH
3
2.6
Cyclohexane C
6
H
12
6.4 Propane C
3
H
8
3.7
Deuterium D
2
0.35 Silver perchlorate AgClO
4
3.6
Dichlorodifluormethane CCl
2
F
2
2.7 Stannic iodide Snl
4
6.7
Dichloromethane CH
2
Cl
2
7.8 Sulfur dioxide SO
2
2.1
Dintrobenzene C
6
H
4
(NO
2
)
2
7.8 Sulfur hexafluoride SF
6
2.3
Ethane C
2
H
6
2.6 Toluene C
6
H
5
CH
3
6.8
Ethanol C
2
H
5
OH 3.6 Trinitrobenzene C
6
H
3
(NO
2
)
3
9.0
Ethylene oxide (CH
2
)
2
O 2.5 Water H
2
O1.0
Helium He 0.14 Xenon Xe 3.0
Hexane C
6
H
14
6.6 Xylene C
6
H
4
(CH
3
)
2
7.8
Hydrogen H
2
0.44
 Loading...
Loading...