Installation
40 03-11-24 1003en01
Location
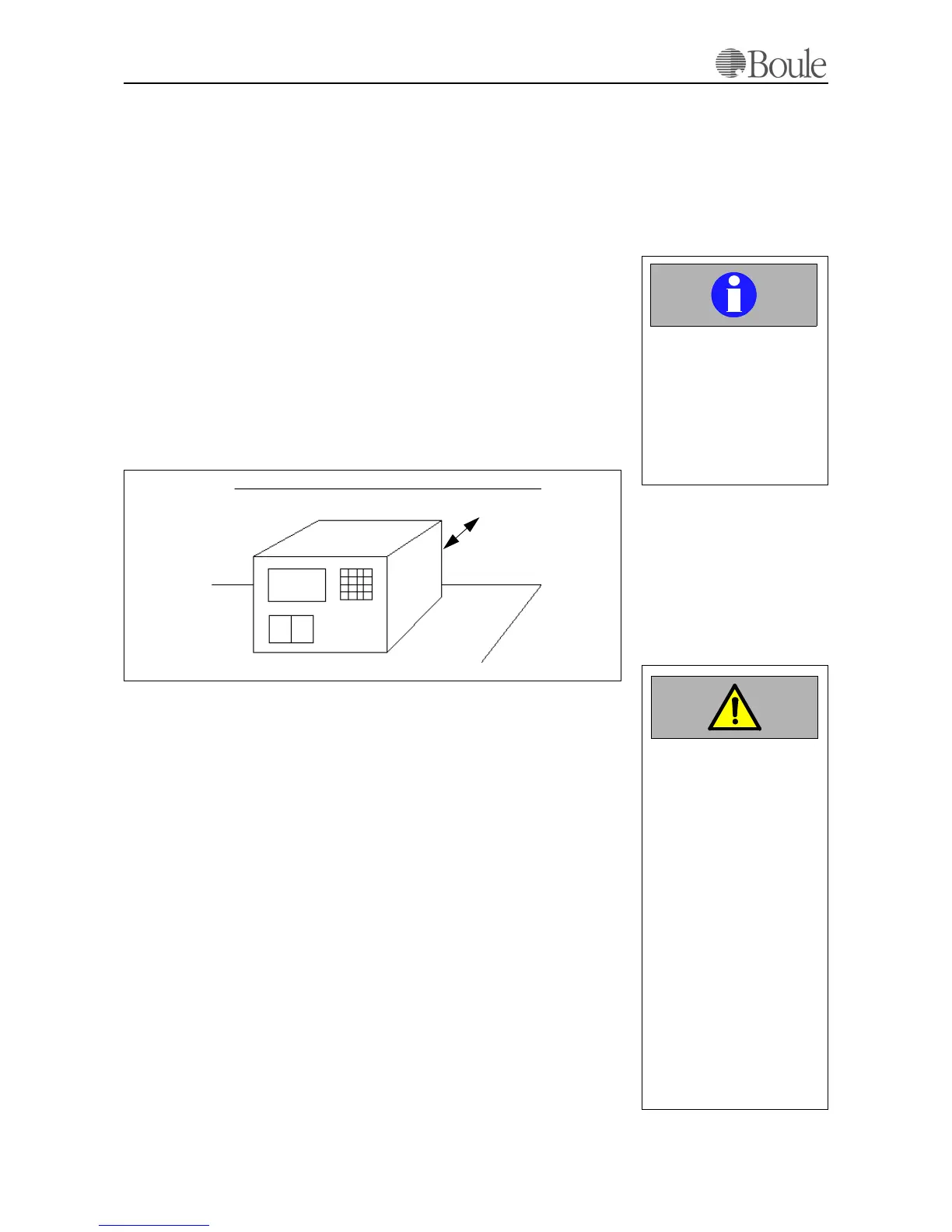
The instrument should be placed on a clean and level table or work station. Avoid
exposure to sunlight. Make sure that the instrument has access to proper ventila-
tion and that at least ca. 10 cm free space must be left at the rear of the analyser,
marked as “A” in the picture below..
Place the instrument with easy access to the mains outlet and the mains cord. In
case that emergency shut-down is needed, due to an obvious malfunction of the
instrument and the instrument needs to be powered off follow the instructions
below:
1. Switch off the instrument immediately by:
Pulling out the mains cord from the mains supply.
2. Contact your authorized distributor’s service department immediately.
The instrument can work correctly within the stated temperature and humidity
range however, to minimize the risk of bacterial growth in the reagent packages,
it is strongly recommended to keep the laboratory at a temperature of approx.
+22°C (72°F).
)
Reagent packages
Reagent packages are preferably placed at the same level as the instrument (e.g.
the same table). In case such conditions cannot be met, place the Diluent con-
tainer at the floor level (maximum1 meter below the instrument level) but keep
the Lyzer container preferably at the same level as the instrument (e.g. the same
table).
Waste connection
The instrument has an open waste outlet and can be connected to a central waste
system within the laboratory. The waste outlet, or container, must always be at a
lower level than the instrument. National and/or local regulations must be fol-
lowed in all cases.
Important
Operating the instrument
in an environment over
+32°C (90°F) increases
service-needs as well as
degradation of sample
specimen.
1178.bmp
A
Warning
Contamination hazard
Because no test method
can offer complete assur-
ance that HIV, Hepatitis B
or C viruses, or other in-
fectious agents are absent,
the waste should be han-
dled at the Biosafety Level
2 as recommended for any
infectious human blood
specimens in Protection of
Laboratory Workers From
Infectious Disease Trans-
mitted by Blood, Body
Fluids and Tissues- 2nd
Edition, Tentative Guide-
lines (1991) Document
M29-T2 promulgated by
the National Committee
for Clinical Lab. Standards
in the U.S.A. (NCCLS)
 Loading...
Loading...