Theory of Operation DF-310E 105
10 Theory of Operation
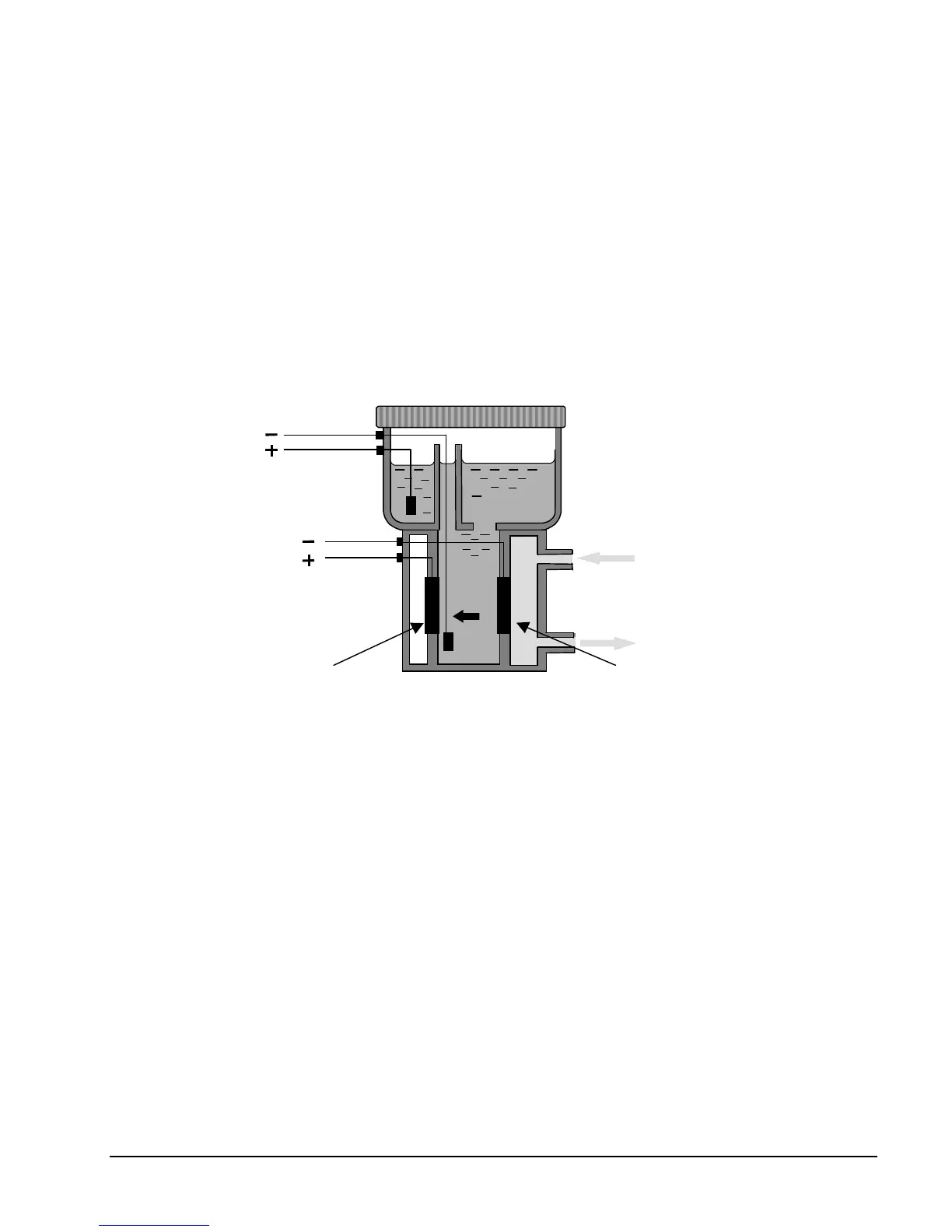
10.1The Oxygen Sensor
The Servomex Coulometric Sensor uses an ambient temperature
oxygen reaction that is non-depleting. The cell produces a current flow
that is determined by the number of oxygen molecules that are reduced
at the cathode. The sensor reaction is driven by 1.3 Volts applied
across the electrodes. The resulting electron flow is measured as a
current that is precisely proportional to the oxygen concentration in the
sample gas.
KOH
4OH
¯
Sample Gas
Cathode
Secondary
Electrodes
Anode
O
2
1.3V Applied
Figure 67: Schematic of Servomex Oxygen Sensor
The cathode reaction uses 4 electrons from the 1.3 volt circuit, 2 water
molecules from the electrolyte, and 1 oxygen molecule from the
sample gas to generate 4 hydroxyl ions which migrate across the
reaction chamber to the anode:
O2 + 2H2O + 4e
-
4OH
-
The anode reaction consumes the 4 hydroxyl ions and delivers 4
electrons to the circuit, 2 water molecules back to the electrolyte, and
vents one oxygen molecule.
4O H
-
O2 + 2H2O + 4e
-
There is no net change to the electrolyte and no depletion of the sensor
or electrodes.
 Loading...
Loading...