2 3
1
1
HEALTH AND SAFETYINFORMATION
Device SafetyCompliance
The 3M™Attest™ Auto-reader390G complies with the following standards as
demonstrated by the CB Scheme Certificate and test report issued by Underwriters
Laboratories(UL):
•
IEC 61010-1(2001) Second Edition. Safety requirements for electrical equipment for
measurement, control, and laboratory use - Part 1: Generalrequirements
•
IEC 61010-2-010(2003) Second Edition. Safety requirements for electrical equipment
for measurement, control, and laboratory use - Part 2-010: Particular requirements for
laboratory equipment for the heating ofmaterials
The 3M™Attest™ Auto-reader390G is listed as Laboratory equipment and carries the
UL mark with adjacent indicators “C” and “US” based on compliance to the standards UL
61010-1and CAN/CSA 22.2No.61010-1.
The 3M™Attest™ Auto-reader390G complies with the CE mark related to the Low
Voltage Directive (LVD) 2006/95/EC as confirmed in the Declaration ofConformity.
The 3M™Attest™ Auto-reader390G complies with the RoHS Directive, Directive
2011/65/EU of the European Parliament and of the Council of 8June 2011on
the restriction of the use of certain hazardous substances in electrical and
electronicequipment.
The 3M™Attest™ Auto-reader390G complies with the WEEE Directive, Directive
2012/19/EU of the European Parliament and of the Council of 04July 2012on waste
electrical and electronic equipment(WEEE).
EMCCompliance
The 3M™Attest™ Auto-reader390G complies with the following EMC standards as
confirmed in the Certificate of Compliance generated by3M:
IEC 61326-1Electrical equipment for measurement, control and laboratory use - EMC
requirements - Part 1: Generalrequirements
The 3M™Attest™ Auto-reader390G complies with the EMC requirements of the CE
mark EMC Directive2004/108/EC.
The 3M™Attest™ Auto-reader390G complies with the Australian and New Zealand
electrical safety and electromagnetic compatibility requirements as confirmed in the
Supplier’s Declaration of Conformity that is linked to the Australian/New Zealand RCM
(Regulatory ComplianceMark).
Note: This equipment has been tested and found to comply with the limits for a Class A
digital device pursuant to Part 15, Subpart B, of the FCC Rules. These limits are designed
to provide a reasonable protection against harmful interference when the equipment is
operated in a commercial environment. This equipment generates, and can radiate radio
frequency energy and, if not installed and used in accordance with the instruction manual,
may cause harmful interference to radio communications. Operation of this equipment
in a residential area is likely to cause harmful interference in which case the user will be
required to correct the interference at their ownexpense.
This Class A digital device meets all requirements of the Canadian Interference-Causing
EquipmentRegulations.
Class 1LEDProduct
An investigation was conducted on the 3M™Attest™ Auto-reader390G per the
requirements of IEC 60825-1Ed. 1.2(A2:2001). The 3M™Attest™ Auto-reader390G is a
Class 1LED product with Class 1InternalRadiation.
The LED apertures are located at the bottom of each Incubator/Reader well perpendicular
to the opening of eachwell.
NOTE: Class 1products are safe for unprotected exposure of theeyes.
Caution - Use of controls or adjustments or performance of procedures other than
those specified herein may result in hazardous radiationexposure.
Note: The use of the term “controls” in the above caution statement as required by
IEC 60825-1Ed. 1.2(A2:2001) refers to mechanisms by which the 3M™Attest™
Auto-reader390G may be controlled and not to the use of biological indicatorcontrols.
EXPLANATION OF PRODUCT AND PACKAGING
LABELSYMBOLS
Attention - Refer to the instructions foruse
Waste Electrical and Electronic Equipment (WEEE) and EU Battery Directive.
This symbol indicates that both the device and the lithium ion battery
contained therein need to be disposed of properly.
UL Listed to US and Canadian SafetyStandards
Mark of Conformity to EuropeanDirectives
Directcurrent
Compliant to all applicable ACMA regulatory arrangements(RCM)
Manufacturer
Catalognumber
Authorized representative for the EuropeanCommunity
Serialnumber
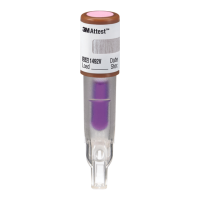
The 3M™Attest™ Auto-reader390G is not designed to incubate
3M™Attest™ 1264EO Biological Indicators, which have a sterilant
entry port in the center of the top of the cap. The 3M™Attest™
Auto-reader390G is designed to incubate and read only 3M™Attest™
Rapid Readout Biological Indicators for EO, catalog number 1294. Other
biological indicators (BIs) are not compatible with this device and cannot
beused.
Li
REF
1294
PREFACE
Manual RevisionHistory
Date Revision Reason For Revision
November 2012 A Initial Issue
February 2013 B
To clarify, in the Indications for Use section, that
Attest™1294Biological Indicators are used to
monitor the ethylene oxide sterilization process.
July 2013 C
To revise: the Warranty statement; and the WEEE
symbol and associated explanatory text.
ContentDisclaimers
PictorialDisclaimer
Sample printouts, graphics, displays and screens are for information and illustration
purposes only, and shall not be used for clinical or maintenance evaluations. Data shown
in sample printouts and screens do not reflect actual patient names or testresults.
HardwareDisclaimer
The 3M™Attest™ Auto-reader390G hardware specifications are subject to change.
The system images, hardware components and hardware specifications included in
the manual may not match the system installed. Any changes or modifications to the
authorized system installation have been verified as compatible with the functionality
outlined in thisdocument.
INDICATIONS FORUSE
The 3M™Attest™ Auto-reader390G is designed to incubate and automatically read the
3M™Attest™ Rapid Readout Biological Indicator for Ethylene Oxide, 1294, at 37°C for a
final fluorescent result at 4hours.
WARRANTY
The 3M™ Attest™ Auto-reader 390G has a one year limited product warranty. The US
warranty, remedy and limitations are described in the Price Quote form and in the Direct
Price Pages. For countries outside of the US, any warranty is established by the subsidiary
with responsibility for service of the 3M™ Attest™ Auto-reader 390G.
SerialNumber
For easy identification, each 3M™Attest™ Auto-reader390G has a unique serial number
printed on a label found on the back of the unit and displayed on the left side of the upper
row of text on the LCD Display when the user depresses the button. Please record
your serial number in this manual for future reference:___________________.
SAFETY ANDPRECAUTIONS
The 3M™Attest™ Auto-reader390G and its related devices and accessories are
designed to provide safe and reliable service when used according to the instructions
provided. Please read, understand, and follow all safety information contained in the
instructions for use included with the 3M™Attest™ Auto-reader390G and 1294Rapid
Readout Biological Indicator devices prior to use. Use this equipment only for the purpose
described in this Operator’s Manual. Retain these instructions for futurereference.
The unit is designed to be used only with the Power Supply module and Ethernet cable
supplied by 3M. If this product is used in a manner not specified, the protection provided
by the product may beimpaired.
Explanation of Single WordConsequences
Warning: Indicates a hazardous situation, which, if not avoided, could result in
death or seriousinjury.
Caution: Indicates a hazardous situation, which, if not avoided, could result in
minor or moderateinjury.
Warnings and SafetyPrecautions
The following warnings and precautions should be followed to avoid unsafe actions that
could result in personal injury or damage to theinstrument.
WARNING: To reduce the risk associated with hazardous voltage
Use indoors only.
Do not use the equipment if it is not working properly or if it has suffered any damage.
Use only the power supply specified for this product and certified for the country
ofuse.
CAUTION: To reduce the risk of injury or instrument damage
Do not spill liquid onto or into the instrument. Do not immerse the unit in liquid.
Always unplug the 3M™Attest™ Auto-reader390G and allow to cool before cleaning.
Clean external surfaces using only the instructions provided by the manufacturer.
Do not open the instrument housing – there are no user serviceable parts.
The instrument must be returned to the manufacturer for repair.
Allow the 3M™Attest™ Rapid Readout Biological Indicator to cool for the
recommended time period before activating. Activating or excessive handling of the
biological indicator before cooling may cause the glass ampoule to burst.
Wear safety glasses when activating the 3M™Attest™ Rapid Readout Biological
Indicator.
WARNING: To reduce the risk associated with incorrect results
Instrument is to be used by operators familiar with the device, device functionality, and
Operator’s Manual.
Do not place the instrument in environment exposed to sunlight or strong
incandescentlight.
Do not place instrument close to any device that emits a strong electro-magnetic field.
Do not use on a vibrating surface.
Match the cap color of the 3M™Attest™ Rapid Readout Biological Indicator with the
color-coded configuration sticker surrounding the 3M™Attest™ Auto-reader390G
incubation wells.
Do not remove or change placement of 3M™Attest™ Rapid Readout Biological
Indicator once it is placed into a well.
Do not remove the 3M™Attest™ Rapid Readout Biological Indicator from the
incubation well until the (+) or (-) symbol on the LCD panel indicates the test
iscomplete.
Caution: To reduce the risk associated with incorrect results
To avoid the possibility of the 3M™Attest™ Rapid Readout Biological Indicator
vial absorbing fluorescent residue from a chemical indicator or tape, place the
3M™Attest™ Rapid Readout Biological Indicator vial so it does not come in direct
contact with chemical indicators or tape.
Rendered on 9/12/2013 10:34:26 AM

 Loading...
Loading...