CUBE 30 TOUCH | USER MANUAL
Results:
Acceptance criteria: CV% ≤ 15%
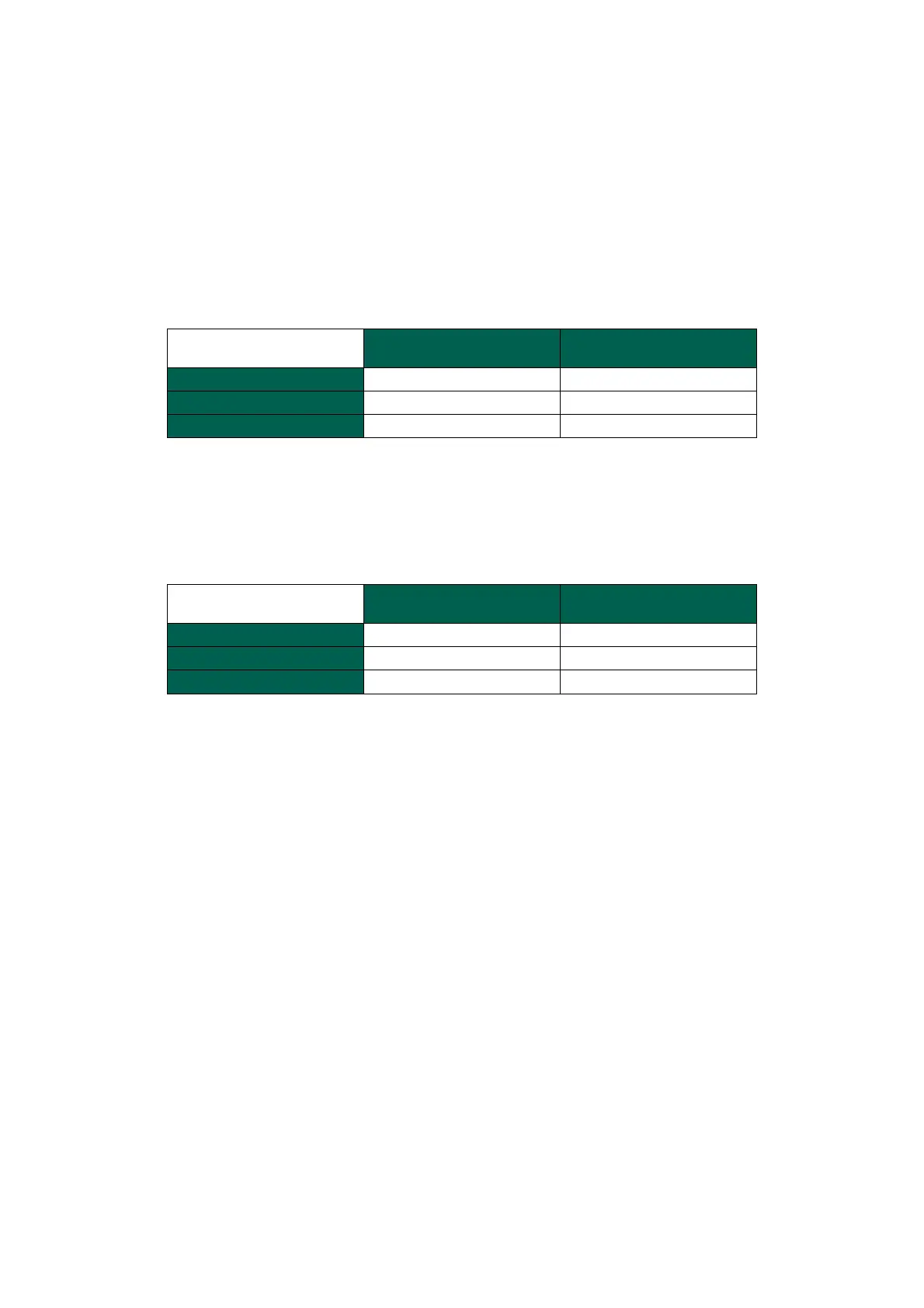
Intra-assay precision (Repeatability):
Means of 30 replicates of each QC blood sample tested on one instrument by a single
operator during 1 working day.
ESR Value (mm/h)
Level 1, Lot 403
ESR Value (mm/h)
Level 2, Lot 403
Intra-assay precision (Within-lab):
Means of 90 replicates of each QC blood sample tested on one instrument by a single
operator during 3 working days spanning the open-vial stability of the control.
ESR Value (mm/h)
Level 1, Lot 403
ESR Value (mm/h)
Level 2, Lot 403
* When the mean value is close to zero, the coefficient of variation will approach infinity
and is sensitive to small changes in the mean. Therefore, CV% is not reported for this
value.
All the values obtained during the precision evaluation experiment fell within the
expected range and confirmed the precision and repeatability of the CUBE 30 TOUCH
instrument.
11.2 CUBE 30 TOUCH correlation
Overview: This study was conducted to verify correlation of the automated Diesse CUBE
30 TOUCH system to the Modified Westergren benchmark method. A CUBE 30 TOUCH
system was evaluated against the manual Fisherbrand™ Dispette™ 2.
Sample Preparation for Modified Westergren:
Blood samples collected in standard 4.0 mL K2EDTA tubes were inverted six to eight
times allowing the air bubble to reach the end of the tube with each inversion. Using a
transfer pipet, aliquots of 1.0 mL of blood were added to the fill line of a Dispette 2
 Loading...
Loading...