VE-1010 Veterinary PC ECG User Manual EMC Information
- 67 -
Appendix 2 EMC Information
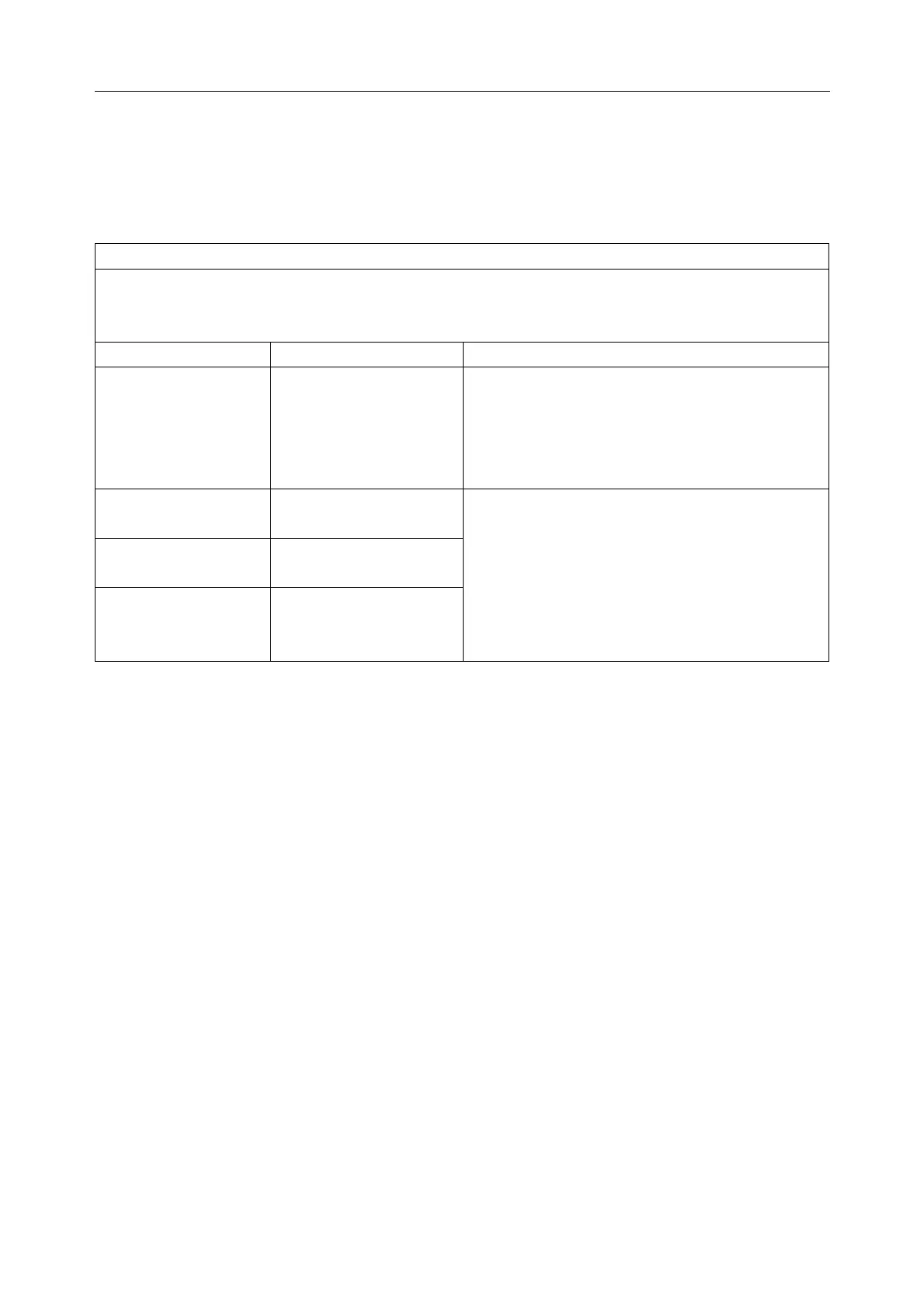
Guidance and manufacture’s declaration - electromagnetic emissions- for all EQUIPMENT
and SYSTEMS
Guidance and manufacture’s declaration - electromagnetic emission
VE-1010 VET PC ECG is intended for use in the electromagnetic environment specified below.
The customer or the user of VE-1010 VET PC ECG should assure that it is used in such an
environment.
Emission test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
VE-1010 VET PC ECG uses RF energy only
for its internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
RF emission
CISPR 11
Class A
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not applicable
VE-1010 VET PC ECG is suitable for use in
all establishments, other than domestic and
those directly connected to the public
low-voltage power supply network that
supplies buildings used for domestic purposes.
 Loading...
Loading...