Basic Electricity 1:5
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
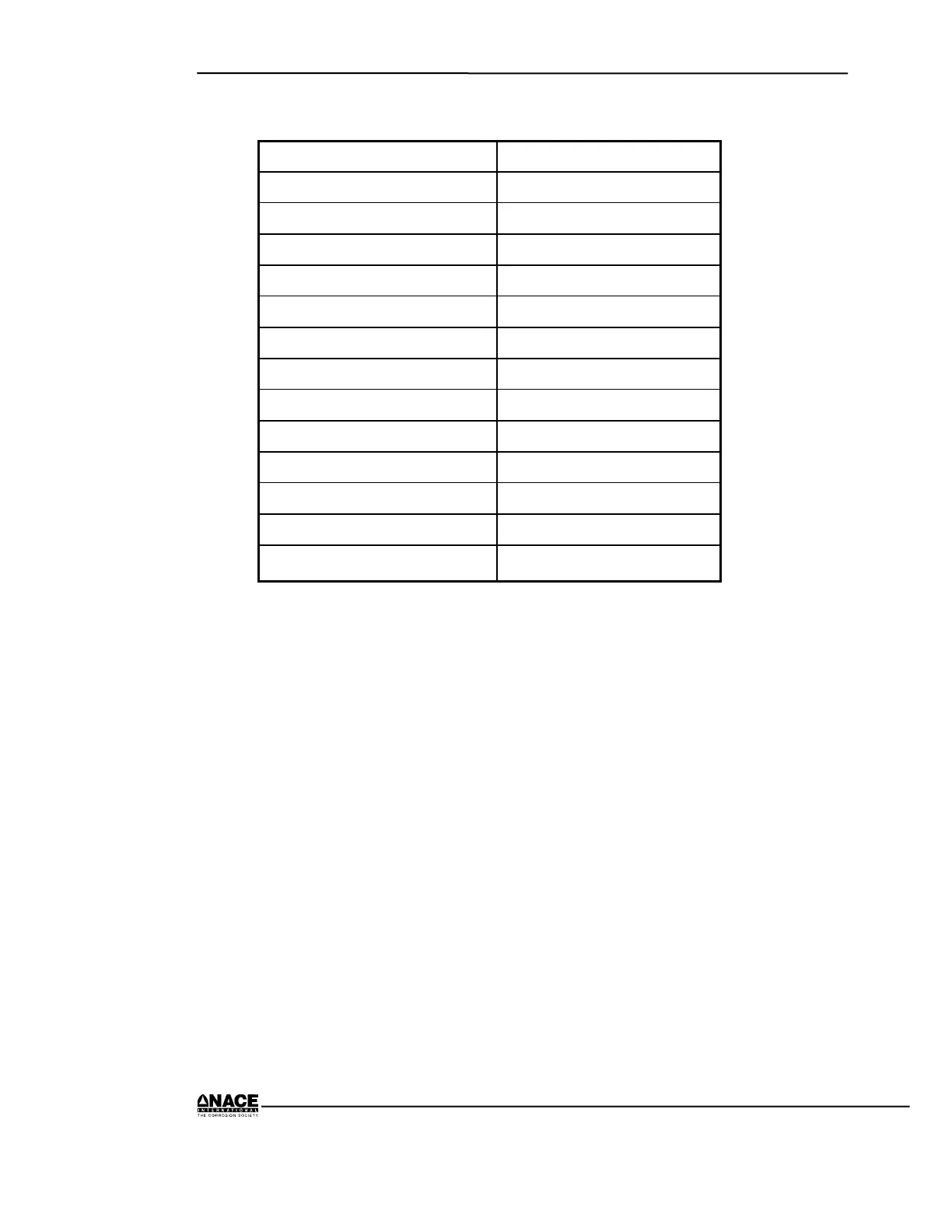
Table 1.1 Typical Resistivity of Common Materials
Material Resistivity (Ω-cm)
Aluminum 2.69 x 10
–6
Carbon 3.50 x 10
–3
Copper 1.72 x 10
–6
Iron 9.80 x 10
–6
Steel 18.0 x 10
–6
Lead 2.20 x 10
–5
Magnesium 4.46 x 10
–6
Zinc 5.75 x 10
–6
Ice 5.75 x 10
8
Rubber 7.20 x 10
16
Water (tap) 3.00 x 10
3
Water (sea) 3.00 x 10
1
Soil (varies) 1.00 x 10
2
to 5 x 10
5
Scientific notation uses exponents where the multiplier 10 is raised
to a power. For example,
1 x 10
2
= 1 x 10 x 10 = 100
1 x 10
-2
= 1 x .1 x .1 = .01
The common unit of resistivity measurement for an electrolyte is
ohm-centimeter. Electrolytes dealt with in corrosion and cathodic
protection includes soils and liquids (water). Since electrolytes do
not usually have fixed dimensions (the earth or a body of water, for
example), resistivity is usually defined as the resistance between two
parallel faces of a cube one cm on each side. Electrolyte resistivities
vary greatly. Some electrolytes have resistivities as low as 30 -cm
(seawater) and as high as 500,000 -cm (dry sand). The resistivity
of an electrolyte is an important factor when evaluating the
corrosivity of an environment and designing cathodic protection
systems. Measurement of electrolyte resistivity is covered in Chapter
5.
 Loading...
Loading...