Basic Chemistry and Basic Corrosion Theory 2:30
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
Conclusions
1. The initial corrosion-cell potential is the difference between the open-
circuit electrode potentials.
2. The more electronegative of the two electrodes is the anode; the more
electropositive of the two cells is the cathode. The corrosion-cell
potential can be estimated using the following expression:
cell potential = potential
cathode
– potential
anode
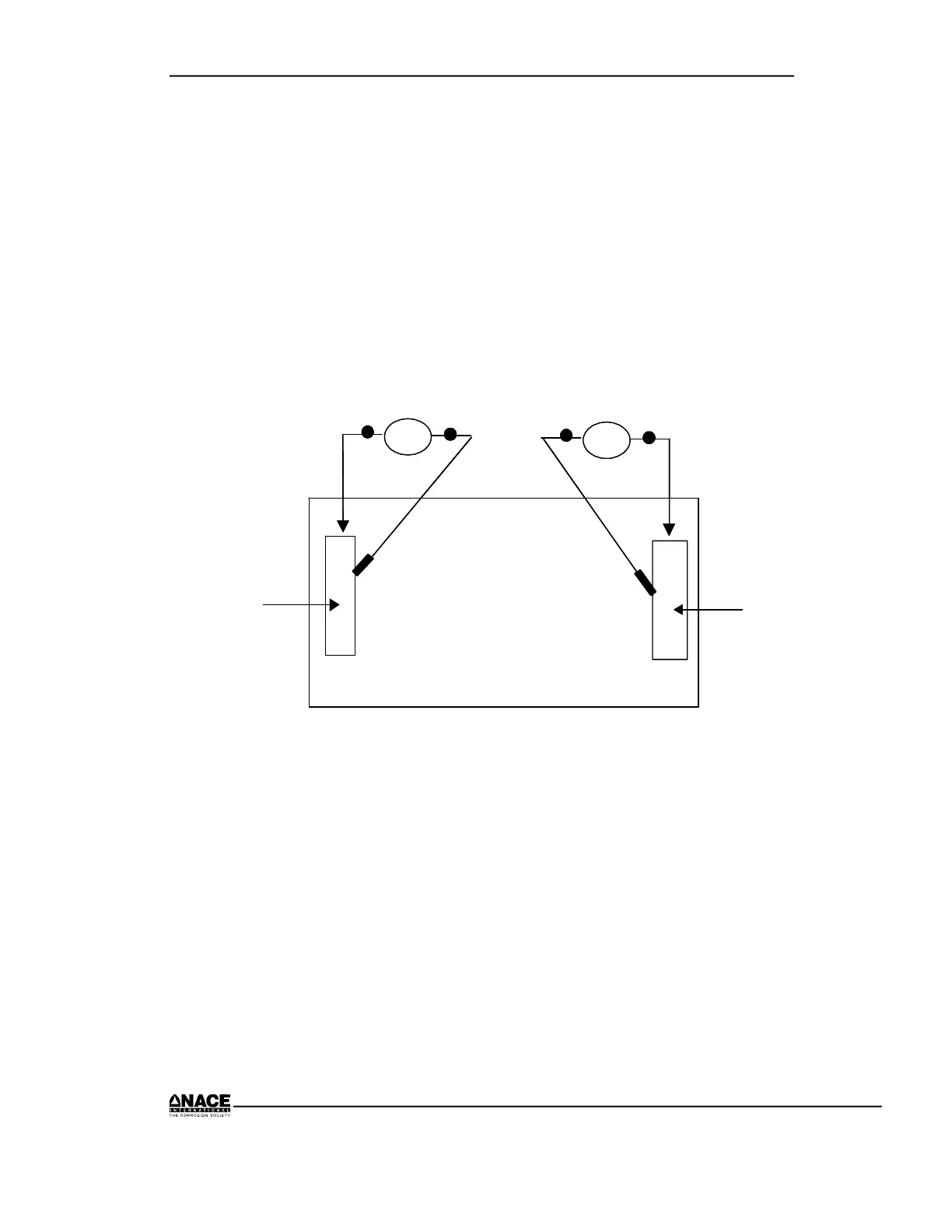
Step B–Potential Measurement
+
–
–+
Copper
Sheet
Steel
Sheet
Tap water in tray
V
V
+
–
–+
Copper
Sheet
Steel
Sheet
Tap water in tray
V
V
1. Connect voltmeter positive lead to copper and connect negative lead to a
copper-copper sulfate reference electrode.
2. Place reference electrode in the water at the copper/water interface.
3. Record the potential.
4. Repeat Steps B1 and B2 for the steel.
 Loading...
Loading...