Basic Chemistry and Basic Corrosion Theory 2:11
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
External Circuit
The external circuit refers to those parts of an electrochemical circuit in
which charge movement is electronic; that is, it involves the movement of
electrons.
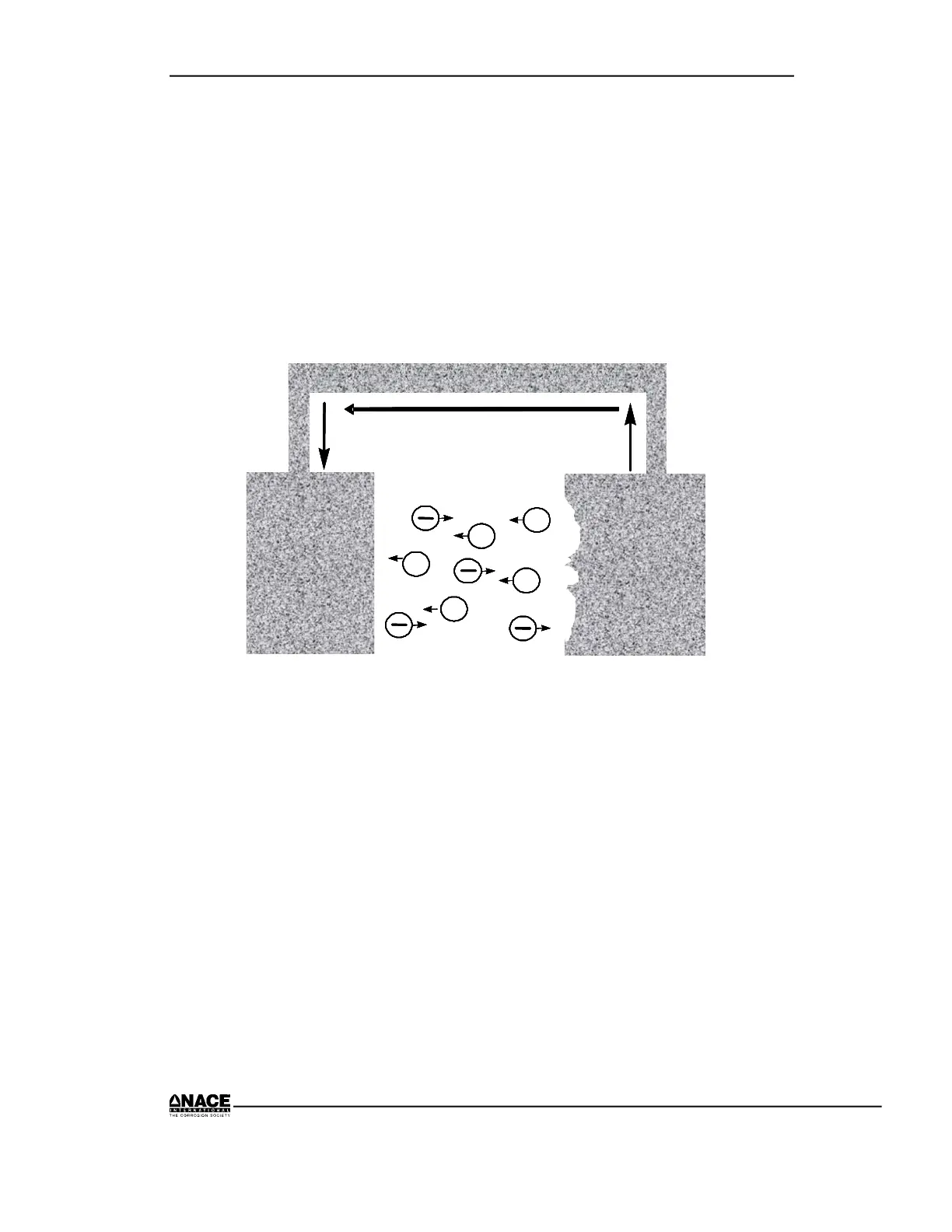
The electric current produced by oxidation and reduction flows through the
electronic path by means of electron movement. The electrons produced in
the oxidation reaction flow from the anode to the cathode to provide
electrons for the reduction reaction to occur. This is shown in Figure 2.10.
+
+
+
+
ELECTROLYTE
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
Direction of Electron Flow
ELECTROLYTEELECTROLYTE
CATHODE
ANODE
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
Direction of Electron Flow
e
-
e
-
e-
e
-
e
-
e
-
e
-
e
-
+
+
+
+
+
ELECTROLYTE
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
Direction of Electron Flow
ELECTROLYTEELECTROLYTE
CATHODE
ANODE
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
e
-
Direction of Electron Flow
e
-
e
-
e-
e
-
e
-
e
-
e
-
e
-
+
Figure 2.10 Electron and Ion Flow
Charge Transfer in the Electrolyte
Movement of charged ions is the mechanism for charge transfer through an
electrolyte as opposed to the flow of electrons in a solid metal conductor.
Positively charged ions (cations) move away from the anode and toward the
cathode. (Note: the ions do not plate out on the cathode.) On the other hand,
negatively charged ions (anions) move toward the anode and away from the
cathode. This charge transfer is called electrolytic current flow. This charge
transfer is shown in Figure 2.10.
Ions are relatively heavy and slow moving. Consequently, electrolytes have
much higher resistivities than metals. This causes a phenomenon called
polarization.
 Loading...
Loading...