Basic Chemistry and Basic Corrosion Theory 2:27
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
Experiment 2.1–Metal Electrode Potentials in Tap
Water
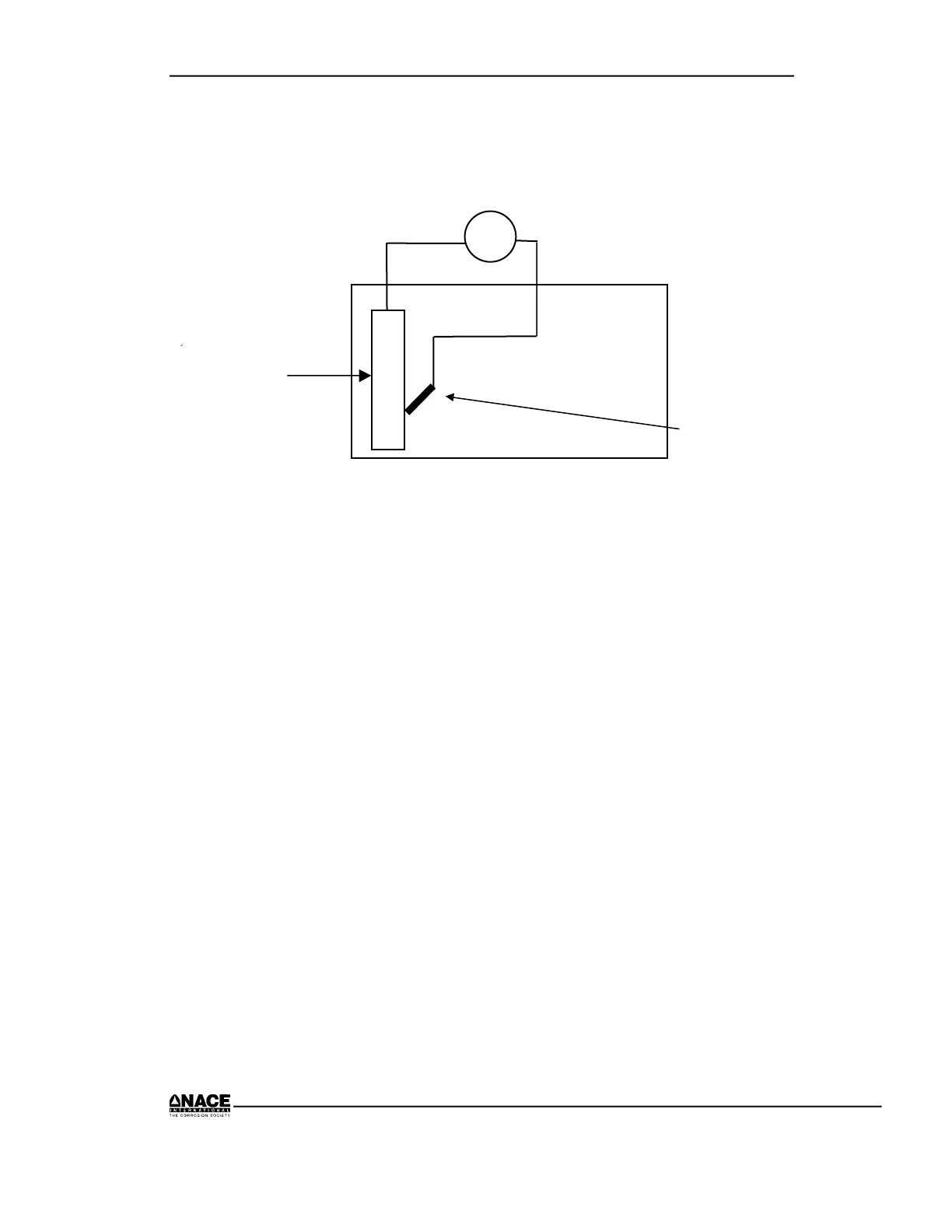
Experiment for Measuring Electrode Voltage (EMF) of Different Metals
with Respect to a Copper/Copper Sulfate Reference Electrode
PROCEDURE
1. Place the copper, steel, zinc, and magnesium into the tray, making sure
they do not touch each other.
2. Add 1-1/2 inch of tap water to the tray.
3. Set the meter to the V DC scale.
4. Connect the meter’s negative lead to the reference electrode.
5. Connect the meter’s positive lead to the metal being tested.
6. Place the reference electrode near the copper and record the copper’s
potential.
7. Place the reference electrode near the steel and record the steel’s
potential.
8. Place the reference electrode near the zinc and record the zinc’s potential.
Reference Electrode
Tap Water in Tray
Metal Sample
V
+
 Loading...
Loading...