Basic Chemistry and Basic Corrosion Theory 2:10
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
Other anode reactions:
Fe
o
Fe
++
+ 2e
–
Al
o
Al
+++
+ 3e
–
Hg
o
Hg
+
+ 1e
–
Cathode Reactions
The chemical reaction that occurs at the cathode, the cathodic reaction, is a
reduction reaction. Reduction is the gain of electrons. The actual cathodic
reaction that occurs will depend on the electrolyte. The following reactions
are the two most common reduction reactions that occur at the surface of
the cathode.
Oxygen Reduction–more common in neutral environments.
2H
2
O + O
2
+ 4e
-
→ 4OH
−
Hydrogen Ion Reduction–more common in acidic environments
H
+
+ e
-
→ H
o
Corrosion never occurs at the cathode of a corrosion cell.
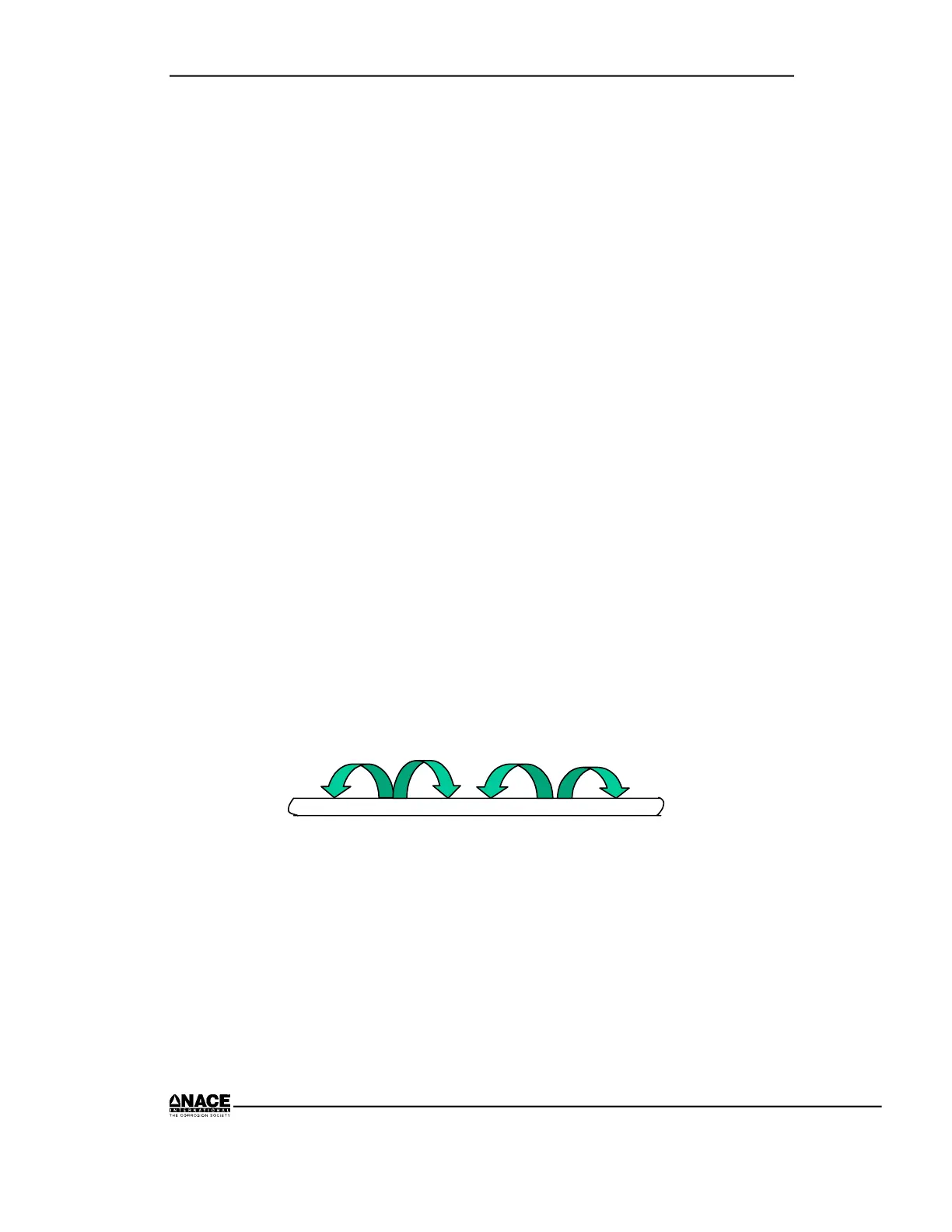
The anode and the cathode can be on different metals or on the same metal
as shown in Figure 2.9.
CATHODIC
ANODIC
ANODIC
Figure 2.9 Typical Local Action Corrosion
Cells on a Structure
 Loading...
Loading...