Basic Chemistry and Basic Corrosion Theory 2:28
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
9. Place the reference electrode near the magnesium and record the
magnesium’s potential.
Results
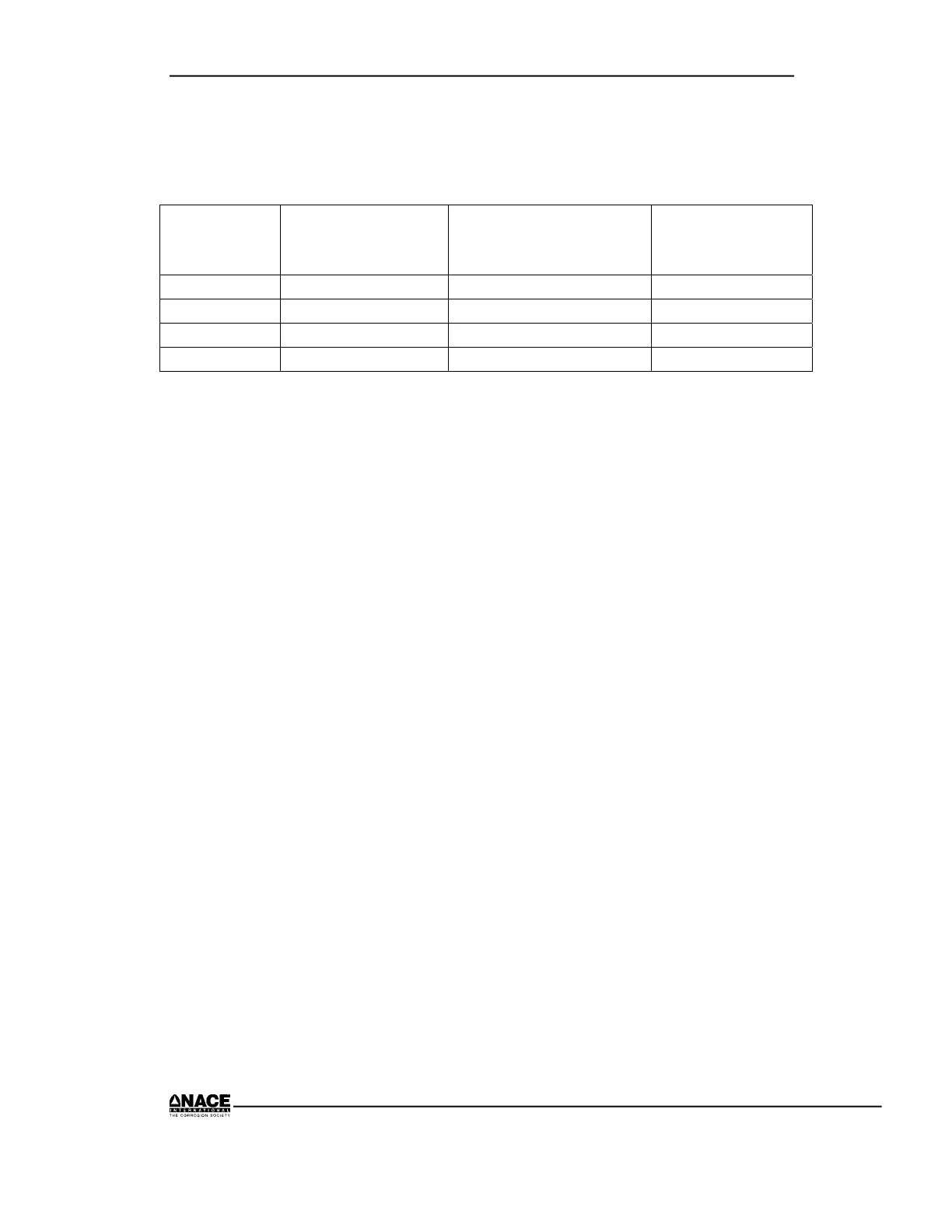
Metal Reference
Electrode
Position
Anticipated Potential
mV/CSE
Actual Potential
Copper Near Copper –100
Steel Near Steel –500
Zinc Near Zinc –1000
Magnesium Near Magnesium –1700
Conclusions
1. Magnesium is more electronegative than copper, steel, or zinc.
2. Zinc is more electronegative than steel or copper.
3. Steel is more electronegative than copper.
4. Copper is more electropositive than steel, zinc, or magnesium.
 Loading...
Loading...