Basic Chemistry and Basic Corrosion Theory 2:32
CP 1 – Cathodic Protection Tester Course Manual
© NACE International, 2000
02/01/05
Results
Metal Sample Potential
Copper
Steel
Conclusions
1. The copper sheet potential is more electronegative than in Part B.
2. The steel sheet potential is unchanged or less electronegative.
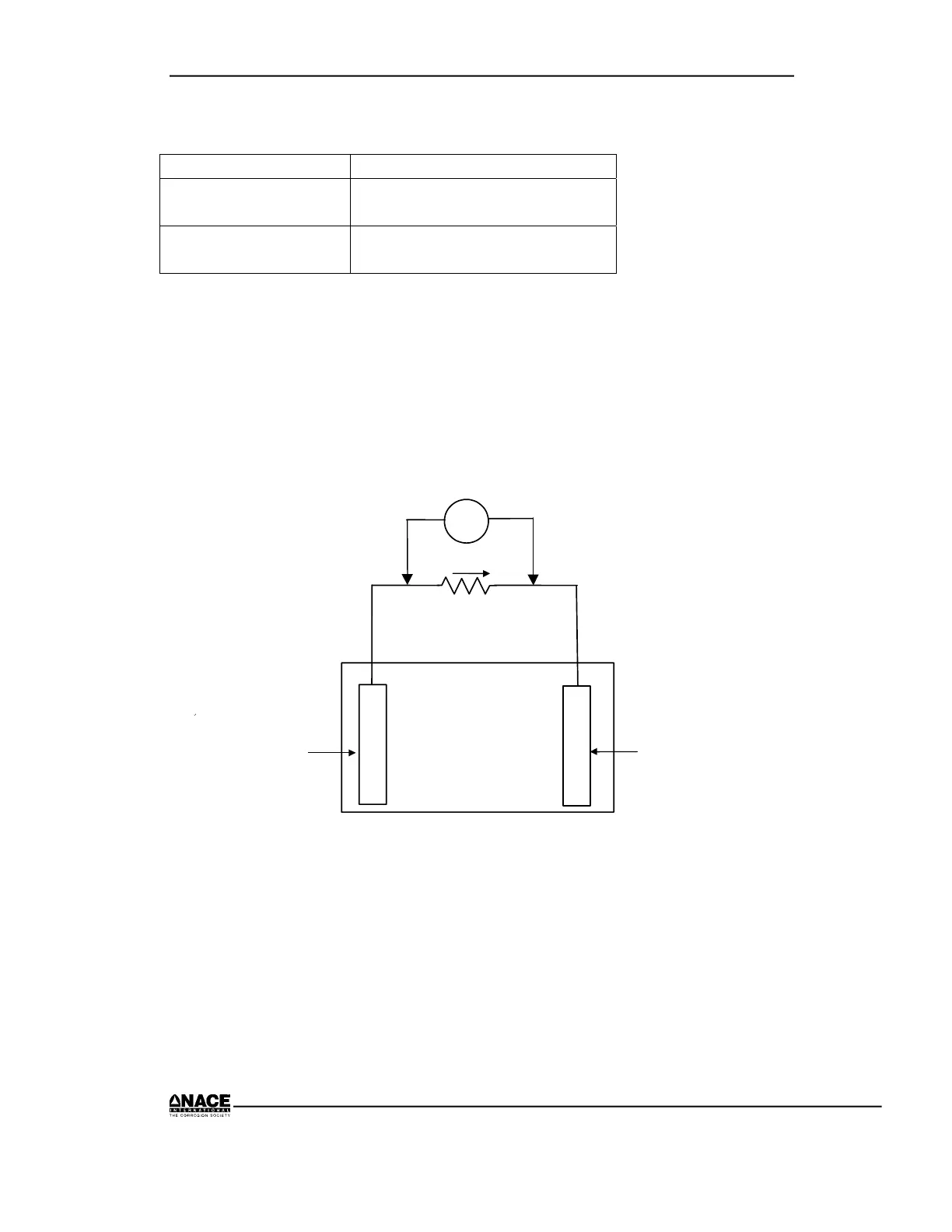
Step D Current Direction in the External Circuit in a
Corrosion Cell
10 Ω
V
Metal Sample
No. 2
Tap Water in Tray
+
Metal Sample
No. 1
1. Connect the meter as indicated across a 10-Ω resistor between the two
metals. See the figure on the next page.
2. Record the voltage drop across the resistor and note polarity.
3. Evaluate the direction of the current.
4. Repeat steps D1 to D3 for the other metal configurations shown in the
table below.
 Loading...
Loading...