Integra 3™ Safety Warnings
8
Research Instruments Ltd
3
Secon 3
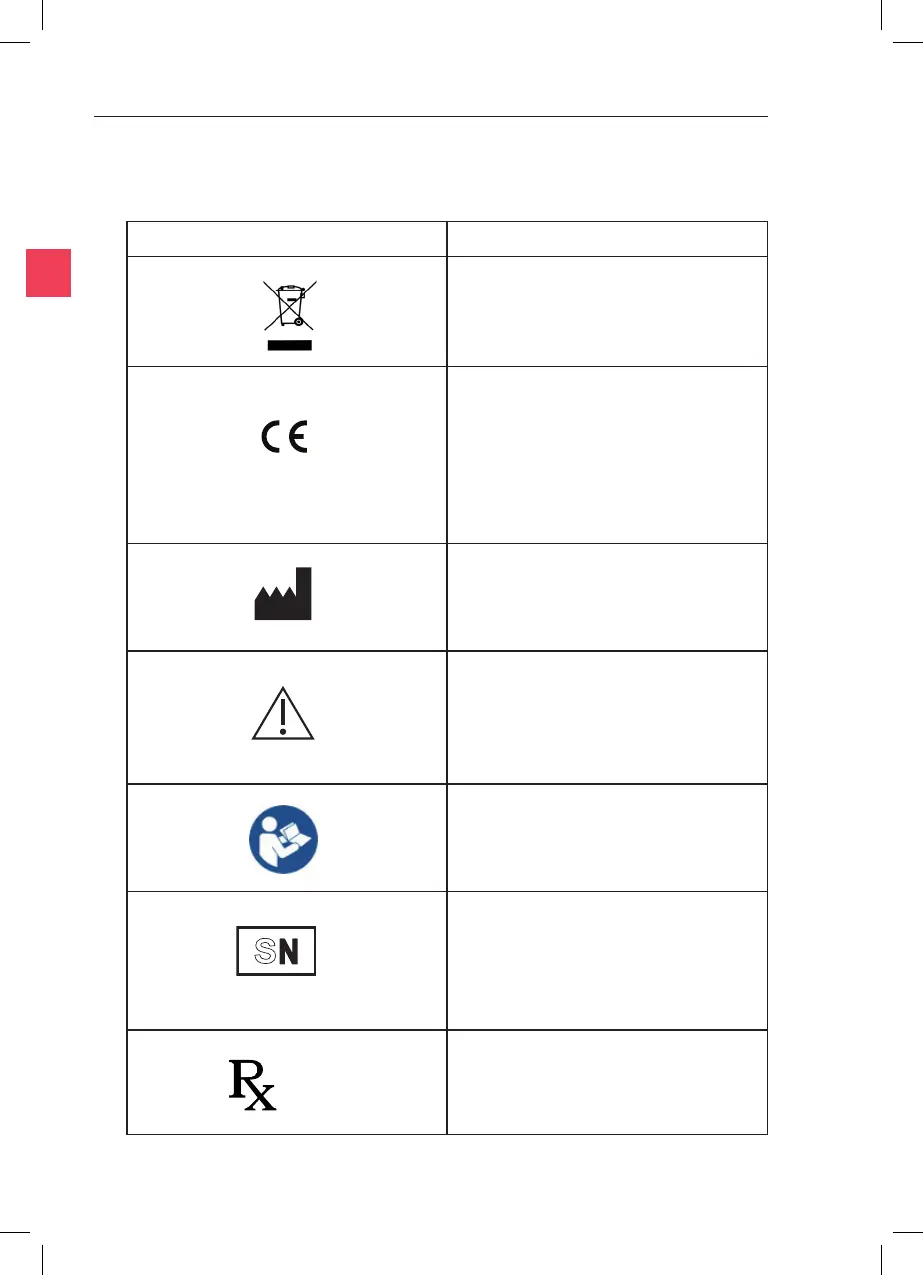
Indicates instrucon for disposal of goods.
In accordance with Annex II of the
European Medical Device Direcve 93/42/
EEC, as amended by Direcve 2007/47/
EC under the supervision of noed body
No.0120, SGS, UK Ltd.
Indicates the medical device
manufacturer.
Indicates the need for the user to consult
the instrucons for use for important
cauonary informaon such as warnings
and precauons that cannot, for a variety
of reasons, be presented on the medical
device itself.
Follow instrucons for use.
The rst four digits are the serial number,
a unique idener assigned to the
product. The last two digits signify the
year of manufacture, eg 5001/13 (this
denotes a unique serial number of 5001
and a year of manufacture of 2013).
Cauon: US Federal law restricts this
device for sale to or on the order of a
licensed healthcare praconer.
Only
S
N
 Loading...
Loading...