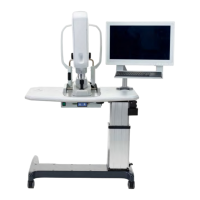
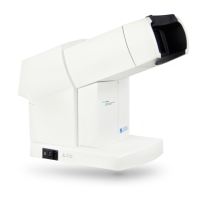
S88 / OPMI Lumera T
Version 8.0
Page 2 G-30-1682-en
About This Manual
These Instructions for Use are part of the delivery scope.
• Please read them carefully before using the device.
• Keep them at the site of use of the device.
• Store them for the entire service life of the device.
• Pass them on to any subsequent owner or user of the device.
Help with orientation
– At the beginning of these Instructions for Use there is a summary of the
different sections; this provides an overview of all the the topics.
– You will also find a detailed table of contents at the beginning of each
section.
– The index facilitates searching via keywords.
Subject to change
without notice
Subject to changes in design and scope of delivery, as well as ongoing
technical development. Printed in Germany.
Scope
These Instructions for Use apply to OPMI LUMERA T with the following stand
and identification:
– S88 floor stand, reference number: 305952-9940-000
Device names in
these Instructions for Use
For the purposes of clarity and improved readability, the device name
"S88 / OPMI Lumera T" has been shortened to "OPMI Lumera T" in these
Instructions for Use.
Trademark
OPMI, OPMI Lumera, Superlux, Invertertube, HaMode and EDIS are
trademarks or registered trademarks of Carl Zeiss Meditec AG or another
company of the ZEISS group in Germany and/or other countries.
Manufacturer's details
Subject to changes in design and scope of delivery, as well as ongoing
technical development. Printed in Germany.
Copyright
© Carl Zeiss Meditec AG 2018
All rights reserved. Reprints and reproductions, in whole or in part, are prohibited.
However, these Instructions for Use may be digitized and archived for internal
use. Access to this data by third parties is not permissible and must be prevented.
Carl Zeiss Meditec AG
Goeschwitzer Strasse 51-52
07745 Jena
Germany
Fax: + 49 (0) 7364 - 20 4823
Email: info.meditec@zeiss.com
Internet: www.zeiss.com/med
 Loading...
Loading...