Seven
™
System User’s Guide | 127
ADVERSE EVENTS
No adverse events related to use of the device were reported during the seven-day trial.
Overall 89% of patients reported no symptoms of irritation and 11% reported at least one
symptom at any insertion site. See Table 13 below for the types of irritation looked for
and reported on during the trial.
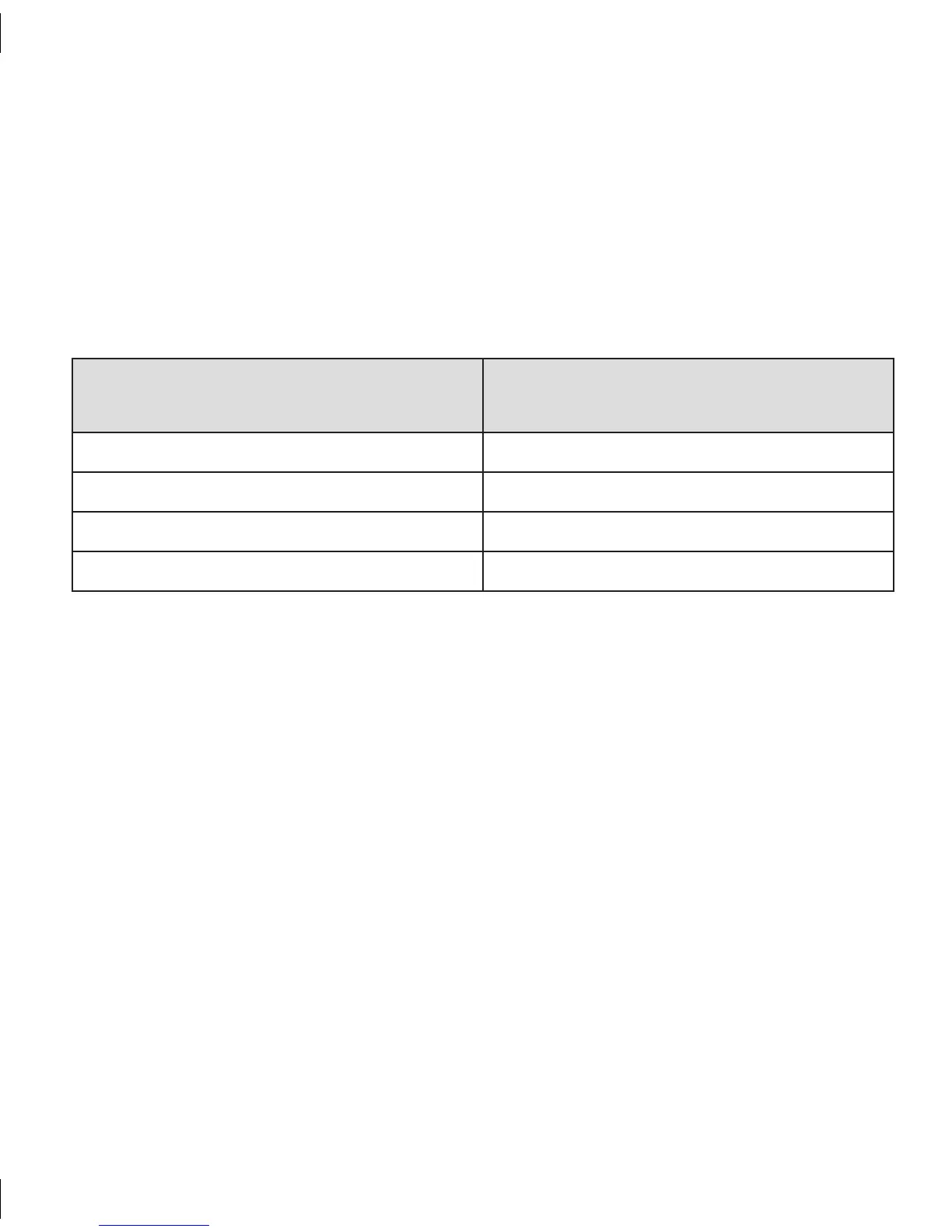
Table 13. Summary of Irritation Events
Symptoms Reported
% of Patient Reported Events at the Sensor Insertion
Sites
Redness (Erythema) 10%
Swelling (Edema) 0%
Bruising 1%
Infection 0%
 Loading...
Loading...