Important Information
14 EchoNous Bladder & Vein User Guide
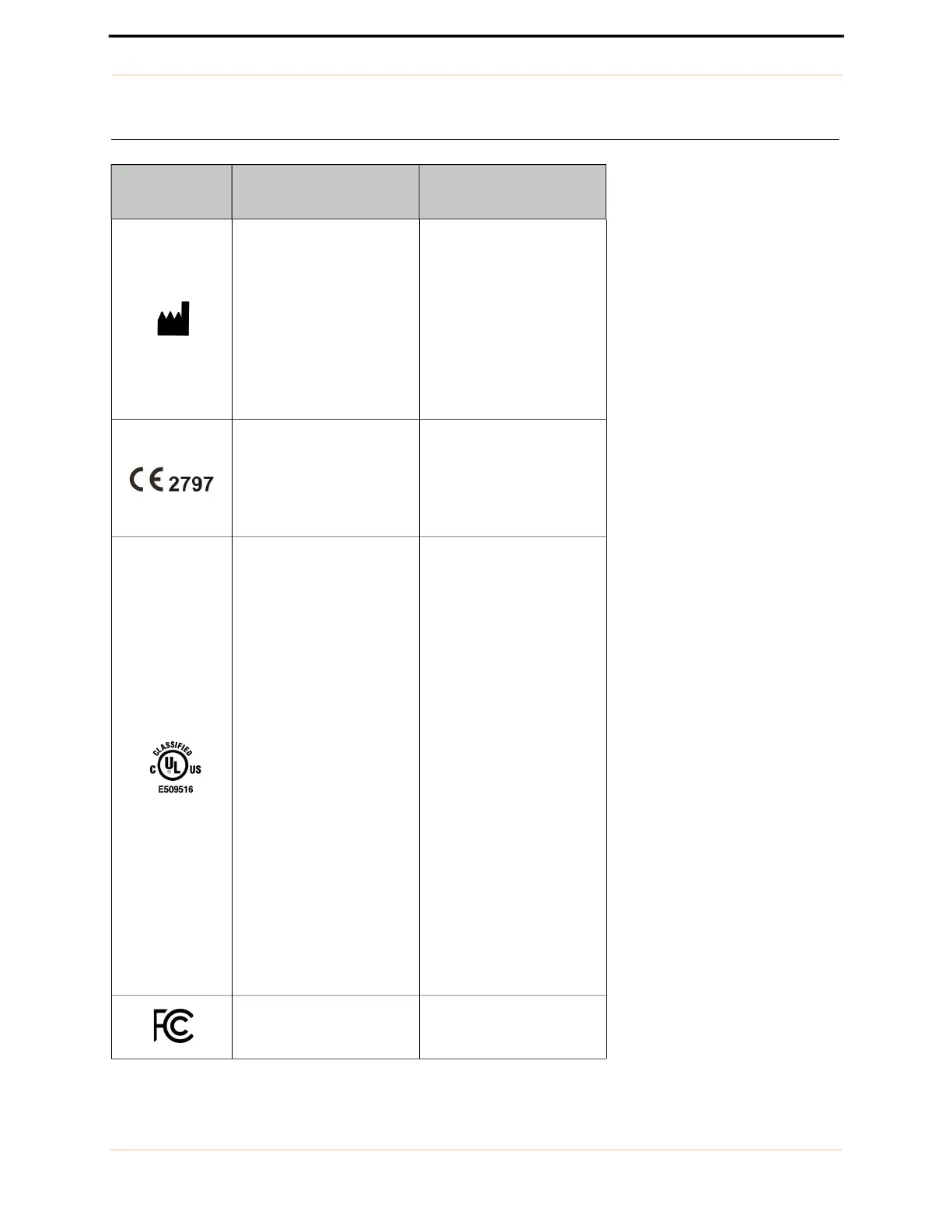
Labeling
Symbol EchoNous Description
SDO Title, Ref. No.,
Standard
Indicates device
manufacturer
Includes name and address
of the manufacturer
Manufacturer
Ref. No. 5.1.1
ISO 15223-1
Medical devices - Symbols to
be used with medical device
labels, labeling and
information to be supplied -
Part 1: General requirements
Manufacturer's declaration
of product compliance with
applicable EEC directives
and the Notified Body
reference number
CE Marking
Ref. Appendix 12
93/42/EEC EU Medical
Device Directive
UL Classified.
Medical - General medical
equipment as to electrical
shock, fire and mechanical
hazards only
in accordance with ANSI/
AAMI ES 60601-1 (2005) +
AMD (2012)/CAN/CSA-C22.2
No. 6060-1 (2008) + (2014).
E509516
Medical - Refurbished
general medical equipment
as to electrical shock, fire and
mechanical hazards only
in accordance with ANSI/
AAMI ES 60601-1 (2005) +
AMD (2012)/CAN/CSA-C22.2
No. 6060-1 (2008) + (2014).
E509516
None
Tested to comply with FCC
standards
None
 Loading...
Loading...