D
IRECTION 5771498-100, REVISION 6 VENUE™ SERVICE MANUAL
1-26 Section 1-1 - Overview
PRELIMINARY
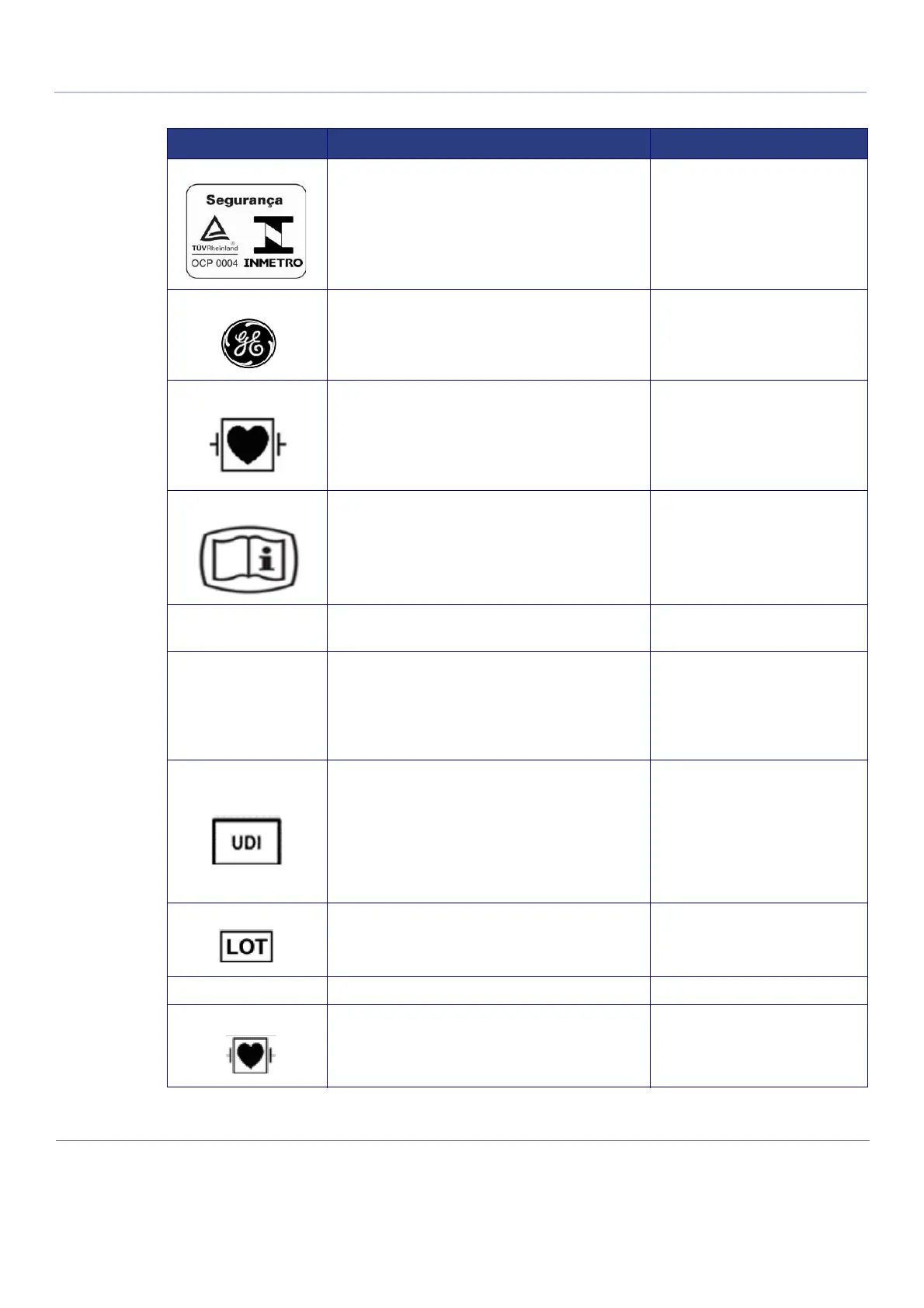
Brazil InMetro Symbol (Pending Approval)
GE Logo
Type CF Defib-Proof Applied Part (heart in the box with
paddle) symbol is in accordance with IEC 60878-02-06.
on ECG module, near ECG
patient cable connector
This device is delivered with Electronic Instructions for
Use (eIFU). This electronic IFU can be downloaded from
the Internet. A paper copy Instructions for Use can be
ordered at no additional cost.
Rating plate or e-Label.
Type/Class
Label
Used to indicate the degree of safety or protection. Rear Panel.
Assembled in X
Purpose: identify the customs country of origin of the
material (x is a country name)
Note: When the Assembled in X statement is not shown
on the label, this indicates that the Customs country of
origin is the same as the country of the legal
manufacturer.
Every system has a unique marking for identification, the
Unique Device Identification
(UDI) Label. The UDI label consists of a series of
alpha-numeric characters and barcode which uniquely
identify the Venue system as a medical device
manufactured by General Electric. Scan or enter the UDI
information into the patient health record as required by
country-specific laws.
Rating plate
Batch code. Indicates the manufacturer’s batch code so
that the batch or lot can be identified.
Rating plate or e-Label
P/N Part Number Rating plate or e-Label
Type CF Defib-Proof Applied Part (heart in the box with
paddle)
on ECG module, near ECG patient
cable connector
Table 1-3 Label Icons and Symbols - Description and Location (Continued)
Label Name Description Location
 Loading...
Loading...