18
2.1 Testing Summary
This section of the document applies to those procedures required to execute the technologist role in the QC of the
Xpress Digital Mammography System.
This section provides the following information:
• Test objectives.
• Recommended frequency to perform the test item.
• Required equipment.
• Procedures to perform the tests.
• Performance criteria and corrective action.
• Documentation.
2.1.1 Test Items, Testing Frequency and Action Criteria
z
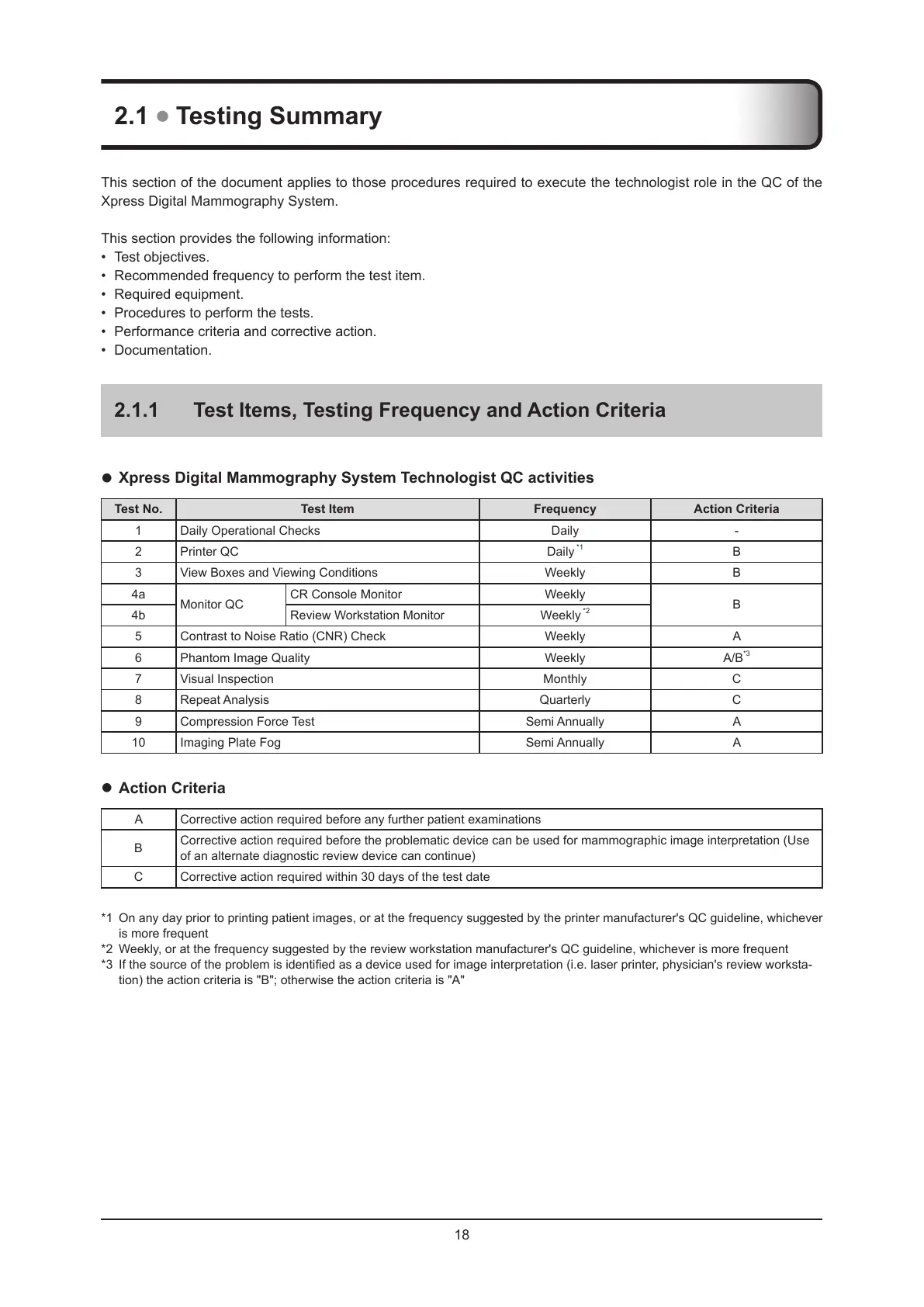
Xpress Digital Mammography System Technologist QC activities
Test No. Test Item Frequency Action Criteria
1 Daily Operational Checks Daily -
2 Printer QC Daily
*1
B
3 View Boxes and Viewing Conditions Weekly B
4a
Monitor QC
CR Console Monitor Weekly
B
4b Review Workstation Monitor Weekly
*2
5 Contrast to Noise Ratio (CNR) Check Weekly A
6 Phantom Image Quality Weekly A/B
*3
7 Visual Inspection Monthly C
8 Repeat Analysis Quarterly C
9 Compression Force Test Semi Annually A
10 Imaging Plate Fog Semi Annually A
z
Action Criteria
A Corrective action required before any further patient examinations
B
Corrective action required before the problematic device can be used for mammographic image interpretation (Use
of an alternate diagnostic review device can continue)
C Corrective action required within 30 days of the test date
*1 On any day prior to printing patient images, or at the frequency suggested by the printer manufacturer's QC guideline, whichever
is more frequent
*2 Weekly, or at the frequency suggested by the review workstation manufacturer's QC guideline, whichever is more frequent
*3 If the source of the problem is identied as a device used for image interpretation (i.e. laser printer, physician's review worksta-
tion) the action criteria is "B"; otherwise the action criteria is "A"

 Loading...
Loading...