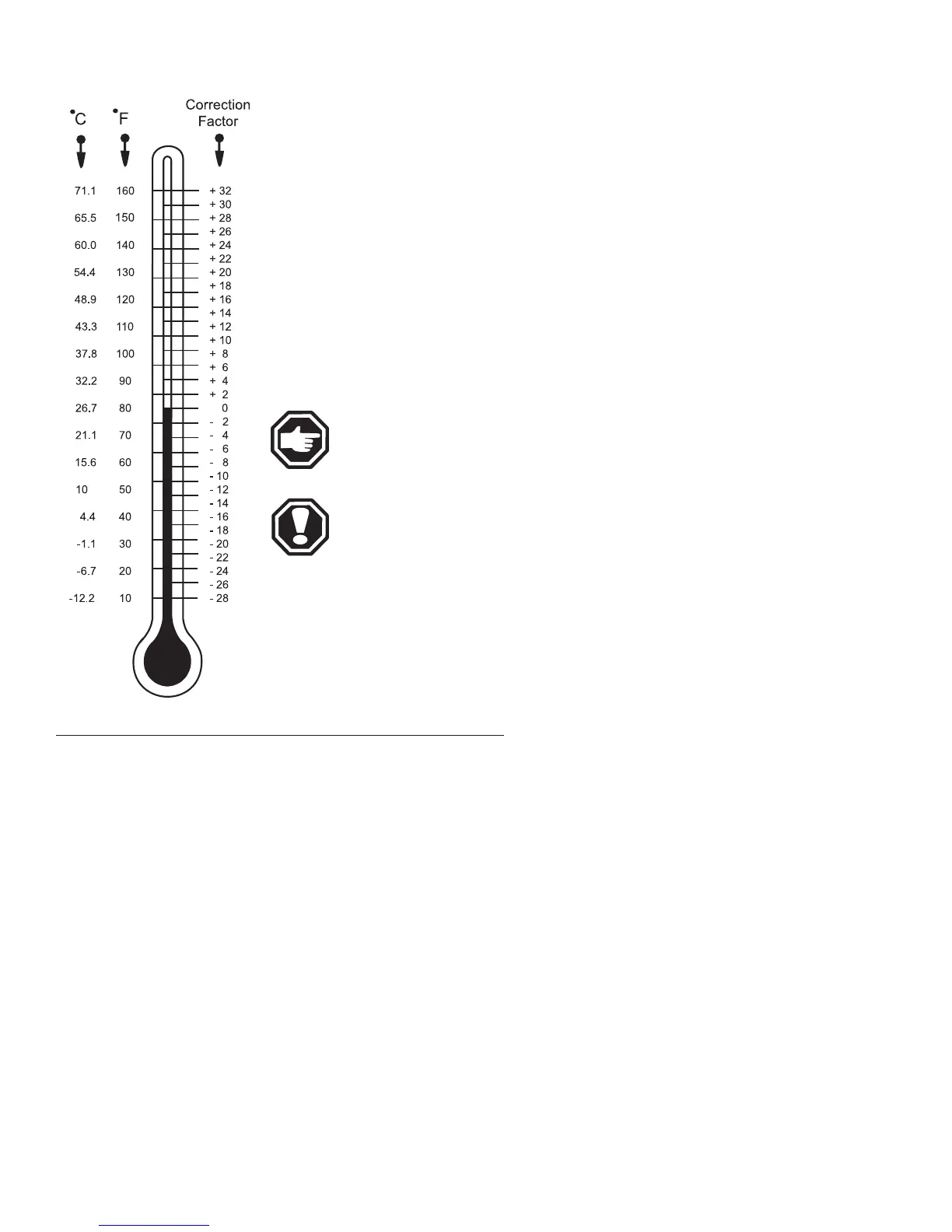
chart. Readings between cells should not vary more than 50 points.
If one cell in a particular battery bank being tested is at a 50% state of
charge while the others are indicating a full charge, charge only that battery
to see if the low cell will come up. At the same time, do not over charge the
“healthy” cells.
If the low cell does not come up after charging, this battery can damage
the rest of the battery bank and should be replaced. An accurate digital volt
meter + - .5% will also give an indicator of the battery’s state of charge.
Another test that can be performed is to place a specific load on the bat-
tery for a predetermined length of time equal to that particular battery’s rat-
ing. This machine is usually an adjustable carbon pile that can vary the load
being applied to the battery(s) while monitoring voltage to see if they will
perform to their specific rated capacities
NOTE: See the chart for temperature compensation.
Liquid levels should be even between the cells of the bat-
tery being tested as it will affect the accuracy of the test.
WARNING: Sulfuric acid in the batteries can cause severe
injury or death. Sulfuric acid can cause permanent dam-
age to eyes, burn skin and eat holes in clothing. Always
wear splash-proof safety goggles when working around the
battery. If the battery electrolyte is splashed in the eyes or
on skin, immediately flush the affected area for 15 minutes
with large quantities of clean water. In case of eye contact,
seek immediate medical aid. Never add acid to a battery
once the battery has been placed in service. Doing so may
result in hazardous splattering of electrolyte.
1. Physical Condition:
Active material flakes off the plates and falls to the bottom of the cell.
This is normal, but sediment accumulation under the plates can short out a cell.
The plate separators fail to insulate positive and negative plates in a cell and the
cell becomes shorted, ruining the battery.
2. Insufficient Electrolyte:
This allows exposed portions of the plates to sulfate rapidly. This reduces
the battery’s ability to accept a charge and the battery capacity is reduced.
Accelerated erosion of the lower portions of the plates occur from higher than
normal acid content due to water loss. Only the water evaporates, not the acid.
The battery also has a higher internal resistance when low on water. Add only
distilled water. Fill each cell to the bottom of the vent well when the battery is
warm. Filling a very cold battery with water to the bottom of the vent well will
cause overspill when the battery warms up and the plates expand. A Battery
Formula For Failure: the battery has a higher internal resistance when low on
water, therefore: high resistance = more heat = shorter battery life!
Electrical Systems - House
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
WINDSOR
8•216
Seven Reasons Why
Batteries Fail
 Loading...
Loading...