Introductory Theory and Terminology
H171804E_14_001 17 / 86
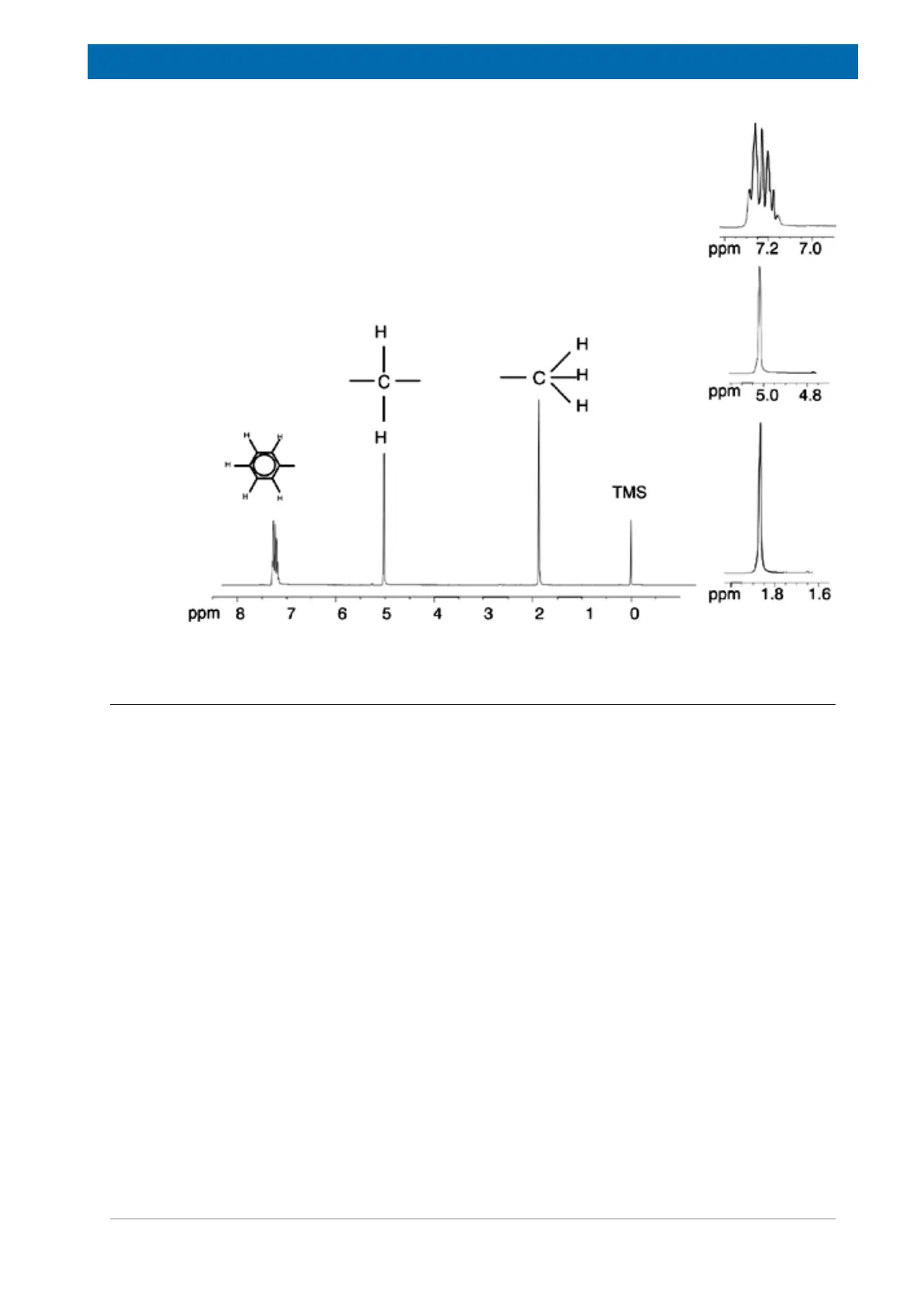
Figure3.10: Proton Spectrum of Benzylacetate
3.6 Proton Spectrum of Ethylbenzene with Spin/Spin Coupling
The description of proton NMR spectra thus far has been greatly simplified by the fact that all
signals, with the exception of those from the benzene ring in benzylacetate, have been
singlets. The structure of the organic compound ethylbenzene and the corresponding proton
spectrum are illustrated in the Ethylbenzene figure and the Ethylbenzene Spectrum figure
respectively. As before, the protons have been labeled as three distinct groups corresponding
to three basic atomic environments.
The most obvious difference between the signals in this spectrum and those of benzylacetate
is the splitting into multiplets. The signal emitted by the CH
3
protons is a triplet and the
signal from the CH
2
protons is a quartet. Note also that the signal positions do not coincide.
The CH
3
protons in benzylacetate emit a signal at 1.85 ppm, while the corresponding CH
3
protons in ethylbenzene emit the triplet signal at 1.25 ppm. This is hardly surprising, because
the two CH
3
groups are in different chemical environments.
The cause of the multiplet splitting is due to an effect known as spin-spin coupling. A full
account of this effect is beyond the scope of this manual and the reader should refer to a
standard NMR text for details. For our purpose a brief outline of the spin-spin coupling should
suffice.

 Loading...
Loading...










