Introductory Theory and Terminology
H171804E_14_001 9 / 86
3 Introductory Theory and
Terminology
NMR is a technique used to analyze the structure of many chemical molecules, primarily
organic compounds. A typical compound might consist of carbon, hydrogen and oxygen
atoms.
In its simplest form, an NMR experiment consists of three steps:
1. Place the sample in a static magnetic field.
2. Excite nuclei in the sample with a radio frequency pulse.
3. Measure the frequency of the signals emitted by the sample.
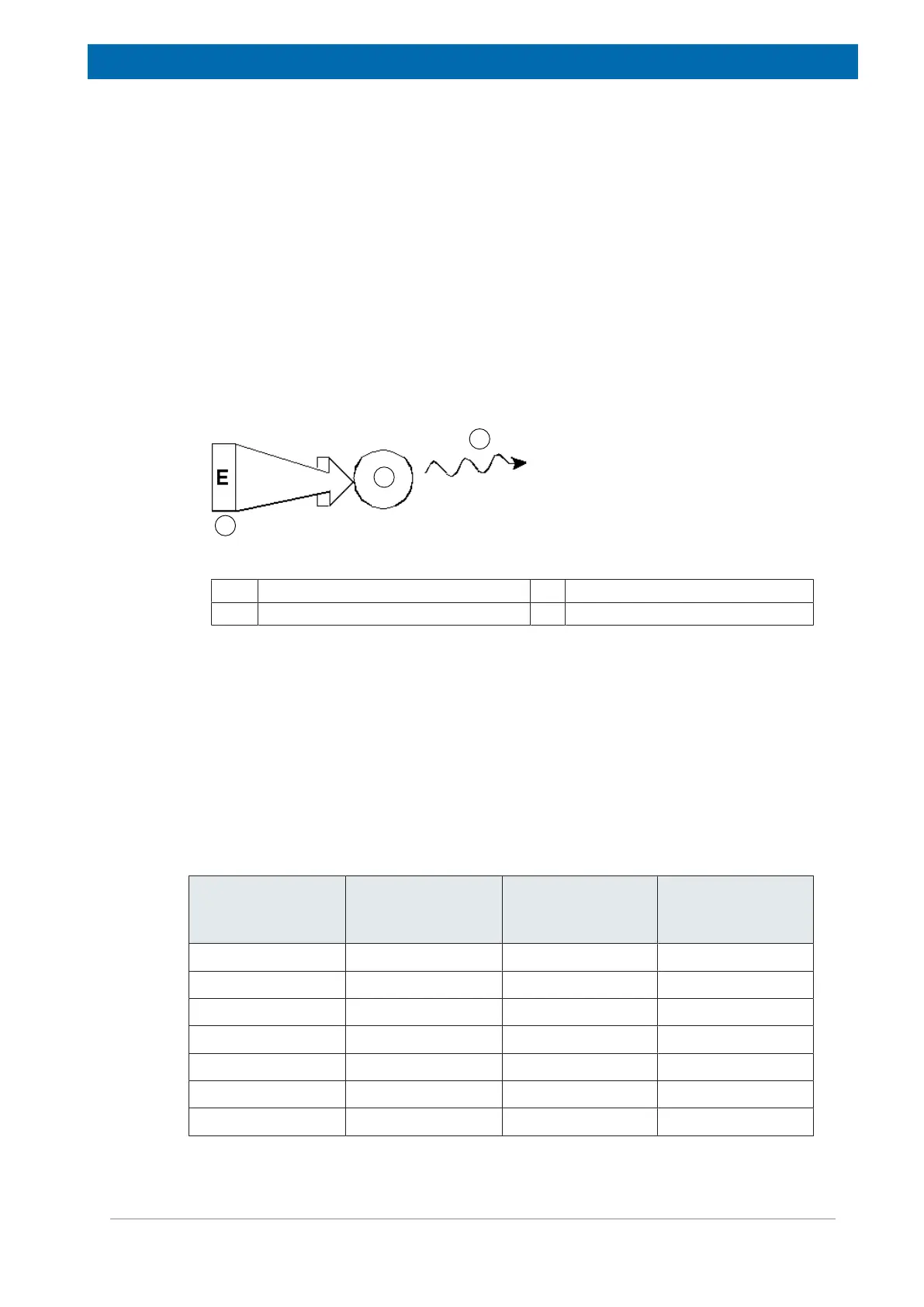
Figure3.1: Excitation and Response
1. Excitation Pulse 2. Atom
3. Emitted Signal
From the emitted frequencies analysts can deduce information about the bonding and
arrangement of the atoms in the sample. The NMR active nuclei in the sample resonate at
different frequencies which are called resonance frequencies. These are the frequencies
emitted by the nuclei when they are excited by the incoming radio frequency pulse. The value
of a resonance frequency depends on two factors:
1) Type of Nucleus:
Every isotope has a particular combination of protons and neutrons in its nucleus. The
nuclear structure largely determines the value of the resonance frequency. Thus every
isotope displays a basic resonance frequency. The
13
C nuclei will have a different basic
resonance frequency compared to that of
1
H etc. Note the large variation in basic resonance
frequencies between different isotopes as listed in the following table:
Nucleus NMR active Basic resonance
frequency
(approx.) [MHz]
Natural
Abundance [%]
1
H yes 500 99.98
2
H yes 77 0.015
3
H yes 533 Traces (ca 10
-18
)
12
C no --- 98.89
13
C yes 126 1.11
35
CI yes 49 75.77
37
CI yes 41 24.23
Table3.1: Table of Data for Various Isotopes (frequencies quoted for an 11.7T magnet)
 Loading...
Loading...