Overview • 89
17-10-07-654-100_01-2020B
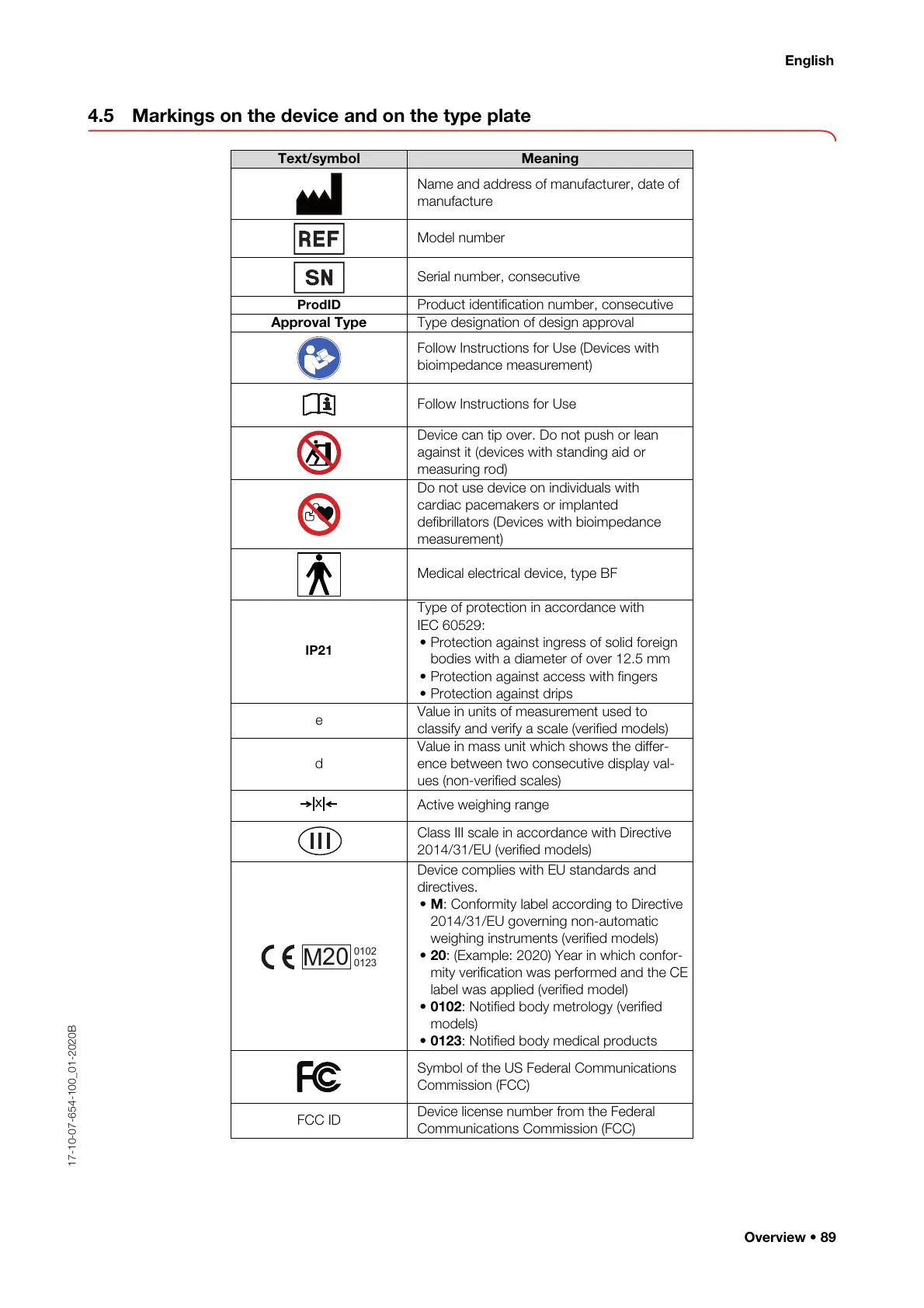
4.5 Markings on the device and on the type plate
Text/symbol Meaning
Name and address of manufacturer, date of
manufacture
Model number
Serial number, consecutive
ProdID
Product identification number, consecutive
Approval Type Type designation of design approval
Follow Instructions for Use (Devices with
bioimpedance measurement)
Follow Instructions for Use
Device can tip over. Do not push or lean
against it (devices with standing aid or
measuring rod)
Do not use device on individuals with
cardiac pacemakers or implanted
defibrillators (Devices with bioimpedance
measurement)
Medical electrical device, type BF
IP21
Type of protection in accordance with
IEC 60529:
• Protection against ingress of solid foreign
bodies with a diameter of over 12.5 mm
• Protection against access with fingers
• Protection against drips
e
Value in units of measurement used to
classify and verify a scale (verified models)
d
Value in mass unit which shows the differ-
ence between two consecutive display val-
ues (non-verified scales)
Active weighing range
Class III scale in accordance with Directive
2014/31/EU (verified models)
Device complies with EU standards and
directives.
• M: Conformity label according to Directive
2014/31/EU governing non-automatic
weighing instruments (verified models)
• 20: (Example: 2020) Year in which confor-
mity verification was performed and the CE
label was applied (verified model)
• 0102: Notified body metrology (verified
models)
• 0123: Notified body medical products
Symbol of the US Federal Communications
Commission (FCC)
FCC ID
Device license number from the Federal
Communications Commission (FCC)
 Loading...
Loading...