9650-1210-01 Rev. R A-1
APPENDIX A
SPECIFICATIONS
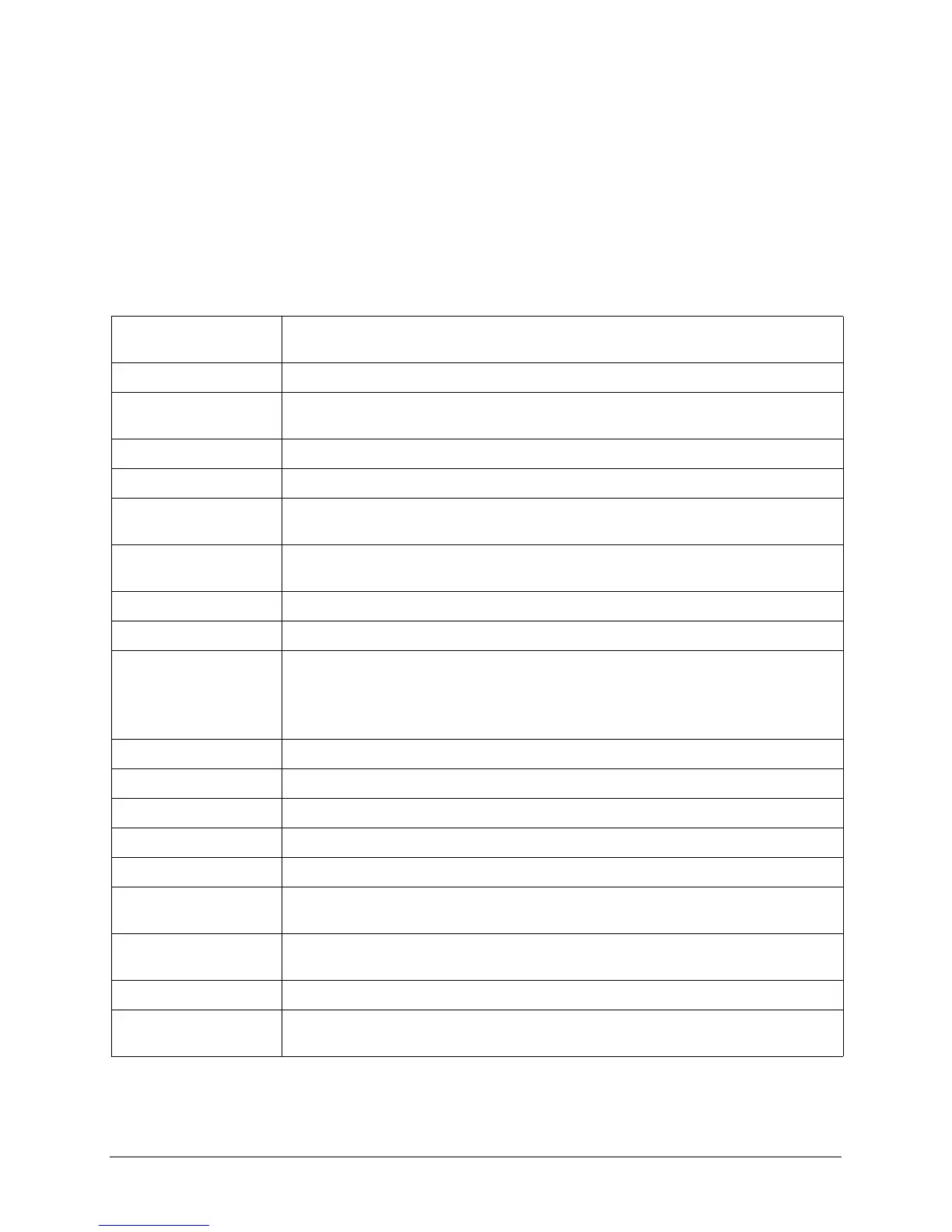
General
Size 5.75 in. high x 13.1 in. wide x 10.5 in. deep
14.6 cm high x 33.3 cm wide x 26.7 cm deep
Weight Approximately 13.2 lbs (5.99 kg)
Power 5 cells, 2 V/cell; wired in series (sealed lead acid battery pack)
3 cells, 4.2 V/cell; wired in series (lithium-ion battery pack)
AC Power 100-120V ~ 50/60 Hz, 220-240V ~ 50 Hz, 220 VA
DC Input (Optional) 10-29 V. 130 W
Device Classification Class I and internally powered per IEC 60601-1.
Class II and internally powered per IEC 60601-1 (DC input ONLY).
Design Standards Meets or exceeds UL 60601-1, AAMI DF-80, IEC 60601-2-4, EN 60601-2-25, and
EN 60601-2-27.
Patient Safety All patient connections are electrically isolated.
Environmental
Temperature: Operating: 0C to 55C (32F to 131F)
Storage Temperature: -20 to 60C (-4F to 140F)
Note: The E Series device may not perform to specifications when stored at the upper or
lower extreme limits of storage temperature and immediately put into use.
Humidity: 5 to 95% relative humidity, non-condensing
Vibration: Mil-Std-810F, Minimum Integrity Test
Shock: IEC 68-2-27, 100 g 6 mS half sine
Operating Pressure: 594 to 1060 mBar
Material Ingress: IP34 per EN 60601-1
Electromagnetic
Compatibility (EMC):
CISPR 11 Class B - Radiated and Conducted Emissions
CISPR 11 Class A - Radiated and Conducted Emissions (DC input only)
Electromagnetic
Immunity
AAMI DF-80, IEC 61000-4-3 to 10 V/m
Electrostatic Discharge AAMI DF-80, IEC 61000-4-2
Conducted
Susceptibility
IEC 61000-4-4, IEC 61000-4-5, IEC 61000-4-6

 Loading...
Loading...