Do you have a question about the Dräger Oxylog VE300 and is the answer not in the manual?
Explains text formatting for instructions, labels, and dialogs.
Defines Dräger's specific use of the term "accessory".
Notes potential differences between document images and actual products.
Lists Dräger and third-party trademarks used in the document.
Specifies the device's purpose and patient conditions for use.
Lists conditions where the device should not be used and suitable environments.
Provides fundamental guidelines for safe product operation and handling.
Details safety precautions specific to the ventilator's functions and potential issues.
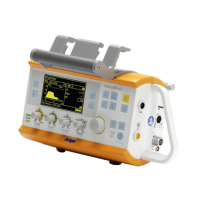
Identifies and describes the parts on the upper side of the main device.
Details the parts of the optional carrying system for the device.
Illustrates and names components of reusable and disposable breathing circuits.
Lists available ventilation modes and additional settings for the device.
Describes the device's physical controls and the layout of the operation screen.
Explains the process of navigating menus, selecting options, and adjusting parameters.
Provides steps for attaching the carrying system and requirements for the oxygen cylinder.
Details how to insert and remove the device's internal rechargeable battery.
Guides on connecting external power sources and gas supplies for operation.
Explains how to connect reusable and disposable breathing circuits to the device.
Covers battery charging procedures and how to estimate device operation time.
Outlines the steps for conducting essential system and breathing circuit tests.
Describes the process for performing CO2 zero calibration and filter tests.
Details the procedure for powering the device on, off, and placing it in standby mode.
Guides on choosing patient categories and ventilation modes for operation.
Explains how to use and manage non-invasive ventilation settings on the device.
Describes how to adjust the inspiratory oxygen concentration using O2/air mix.
Explains the different alarm levels, their visual/audible indicators, and priorities.
Details how to temporarily silence audible alarms and the conditions for reactivation.
Guides on configuring upper and lower alarm limits for various parameters.
Covers settings like screen brightness, breathing circuit type, HME, and ventilation modes.
Explains how to activate Bluetooth connectivity and take device screenshots.
Describes access to password-protected settings like language, date, time, and demo mode.
Details the procedure for resetting all device settings to their original factory defaults.
Explains how to view recorded events, alarms, and device status changes.
Guides on how to retrieve details like software version, serial number, and device ID.
Provides a table listing alarms, their causes, and suggested remedies for resolution.
Explains the meaning and causes of various messages displayed in the alarm field.
Lists errors that can occur during system tests and how to address them.
Details steps for safely disassembling the device and its components for cleaning.
Covers disinfectants, safety, and classification of devices for reprocessing.
Lists recommended cleaning agents, contact times, and temperatures for reprocessing.
Summarizes reprocessing methods and intervals for various device parts.
Highlights critical safety warnings related to performing service and maintenance.
Outlines required inspection intervals and maintenance tasks for the device.
Provides guidance on what to do in case of device malfunction or damage.
Explains procedures for disposing of the device and its rechargeable battery according to regulations.
Lists physical dimensions, weight, screen details, and external ports of the device.
Specifies operating, storage, and transportation temperature, pressure, and humidity ranges.
Details adjustable parameters, ventilation modes, and performance metrics like flow and resistance.
Describes accuracy of measurements, monitoring parameters, and alarm conditions.
Provides information on EMC testing, emissions, and immunity standards compliance.
Covers Bluetooth functionality, separation distances, and IT network connectivity.
Lists order numbers for carrying systems, software options, batteries, and power supplies.
Details various filters, HMEs, NIV masks, and breathing hoses with order numbers.
Lists reusable parts, test lungs, and USB flash drives with their order numbers.
Explains which areas are password-protected and the password for system setup.
| Brand | Dräger |
|---|---|
| Model | Oxylog VE300 |
| Category | Medical Equipment |
| Language | English |