DIRECTION 5750007-1EN, REV. 1 LOGIQ E10 BASIC SERVICE MANUAL
Chapter 1 Introduction 1 - 15
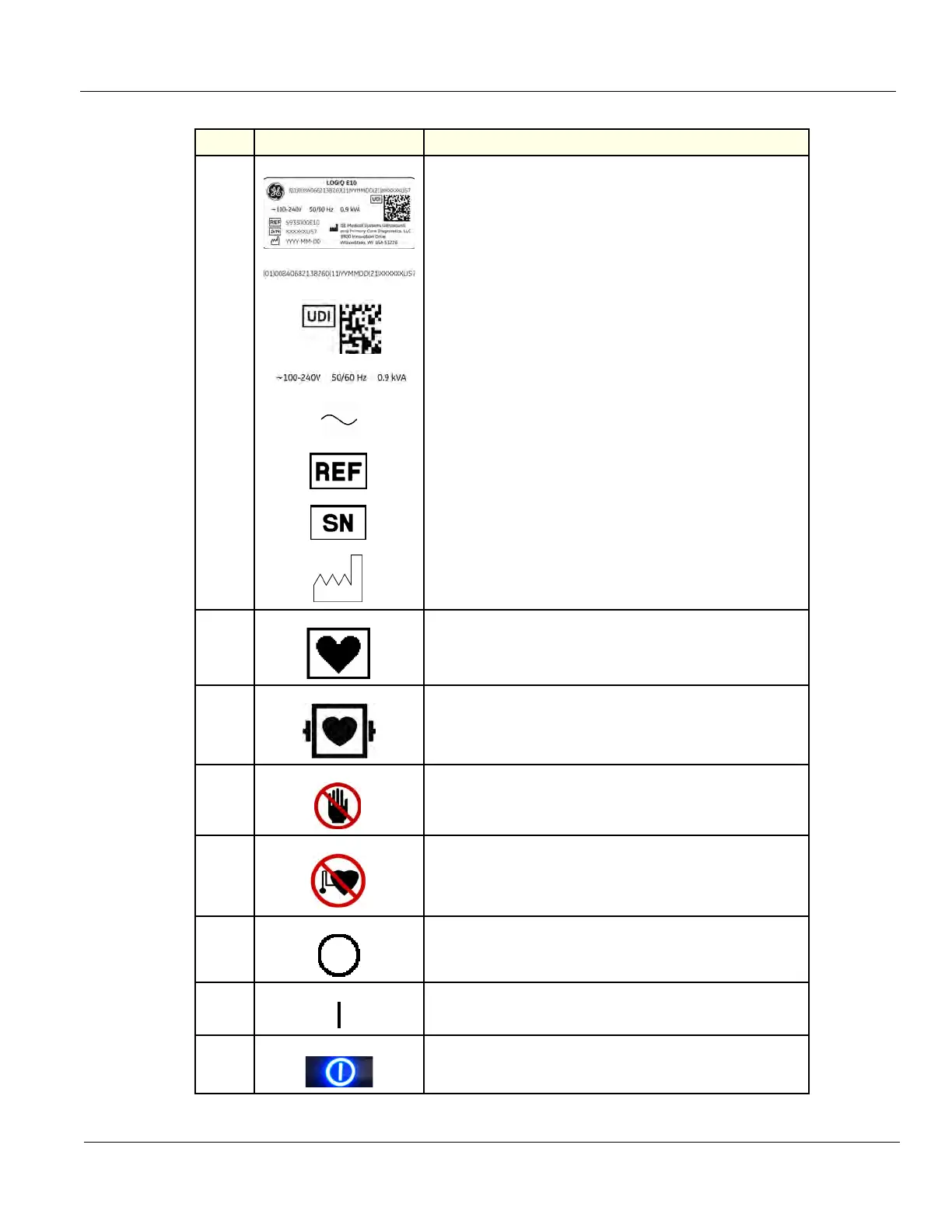
22. Every system has a unique marking for identification, the Unique
Device Identification (UDI) Label. The UDI label consists of a series
of alpha-numeric characters and barcode which uniquely identify the
LOGIQ E9 system as a medical device manufactured by General
Electric. Scan or enter the UDI information into the patient health
record as required by country-specific laws.
UDI Human Readable Label Text: Global Trade Item Number, GTIN,
Manufacturing Date, Serial Number
UDI Symbol and Data Matrix
System Voltage
Alternating Current symbol is in accordance with IEC 60417-5032
Catalog/Model Number. Standard: ISO 7000-2493.
Serial Number. Standard: ISO 7000-2498.
Date of manufacture YYYY-MM. Standard: ISO 7000-2497.
ECG eTRAX needle
Type CF Applied Part (heart in the box) symbol is in accordance with
IEC 60417-5335.
ECG ECG and Probes
Type CF Defib-Proof Applied Part (heart in the box with paddle)
symbol is in accordance with IEC 60417-5336.
Monitor
Arm
DO NOT place a finger, hand or any object on the joint of the monitor
or monitor arm to avoid injury when moving the monitor and monitor
arm.
V Nav ISO 7010 - P007
Volume Navigation Pacemaker Warning
Op Panel “Mains OFF” indicates the power off position of the mains power
breaker.
Op Panel “Mains ON” indicates the power on position of the mains power
breaker.
Op Panel Power On/Off.
Standard: IEC 60417-5010
Table 1-5 Label Icons (Rear of Console)(Continued)
Item Label/Icon Purpose/Meaning
 Loading...
Loading...