Column
Kβ filter
The characteristic x-rays used for x-ray diffractometry generally contain Kα and Kβ x-rays. A substance that passes
Kα x-rays while absorbing most Kβ x-rays to monochromatize the beam is called a Kβ filter.
The mass absorption coefficient (hereinafter referred to as “absorption coefficient”) of an element becomes smaller
as wavelengths become shorter, and vice versa. However, when x-rays with energy greater than the binding energy
between the nucleus and electrons of an element, strike the element, the absorption coefficient suddenly increases
due to the photoelectric effect. This discontinuous point in the absorption coefficient is called an absorption edge.
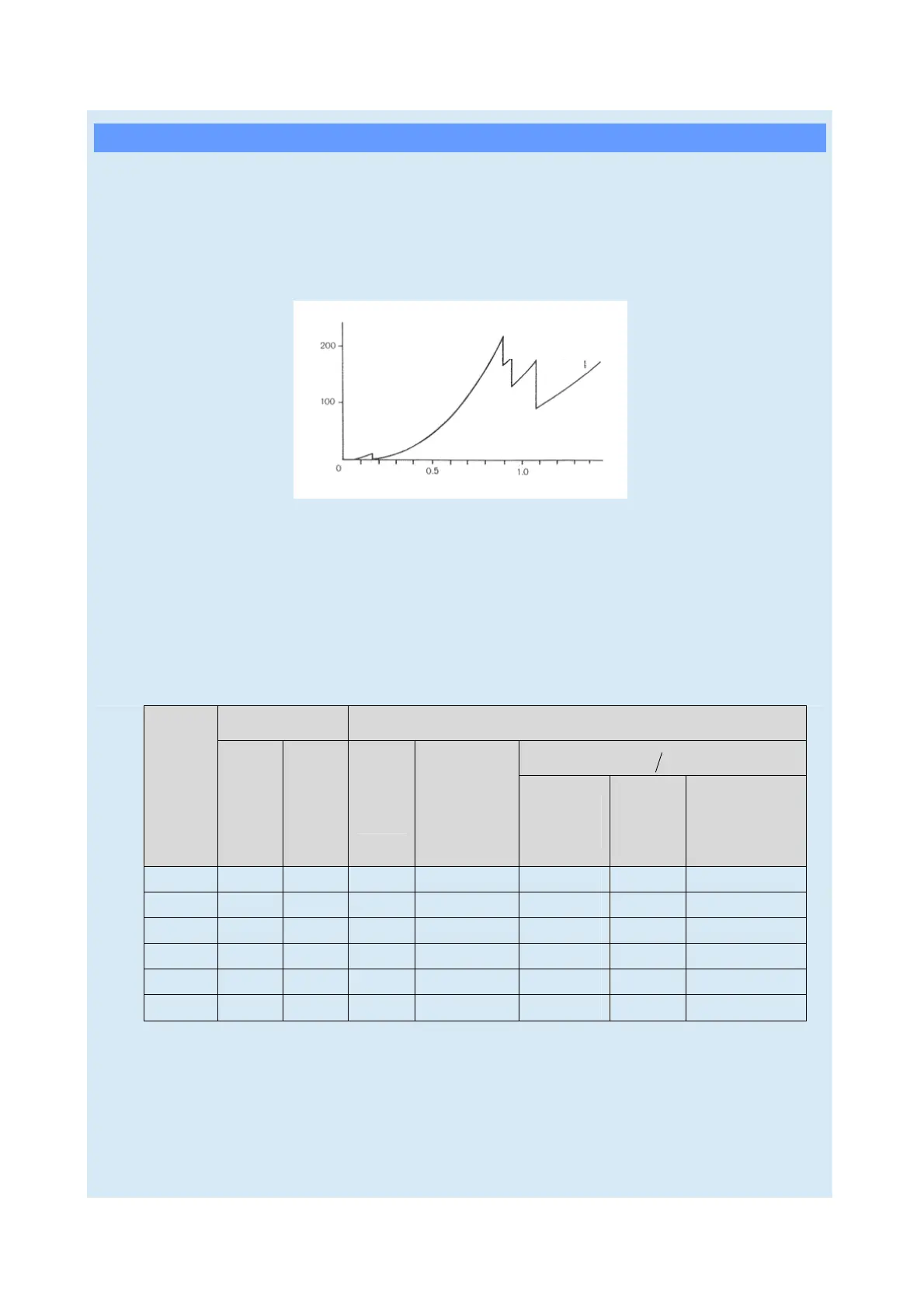
Change in mass absorption coefficient in accordance with wavelength (platinum)
For efficient absorption of Kβ x-rays only, select an element as a Kβ filter with its absorption edge wavelength
located between the Kα x-ray and Kβ x-ray wavelengths. Those elements will have an atomic number one or two
less than that of the target element. The following table shows the target elements commonly used for x-ray
diffractometry and their corresponding filters.
Table Kβ filters
Wavelength (Å) Metals used as a filter
When
100/1
11
=
αβ
KK
II
Target
Kα
1
Kβ
1
Substa
-nces
Wavelength
of
absorption
edge
(Å)
Thickness
(mm)
Mass
per unit
area
(g/cm
2
)
Kα
1
transmissivity
Cr 2.290 2.085 V 2.269 0.011 0.007 63
Fe 1.936 1.757 Mn 1.896 0.011 0.008 62
Co 1.789 1.621 Fe 1.743 0.012 0.009 61
Cu 1.541 1.392 Ni 1.488 0.015 0.013 55
Mo 0.7093 0.6323 Zr 0.689 0.081 0.053 43
Ag 0.5594 0.4970 Rh 0.534 0.062 0.077 41
Mass absorption coefficient
L
I
absorption
edge
L
II
absorption edge
Wavelength (Å)
K absorption
edge
L
III
absorptin
edge
 Loading...
Loading...