50 AU-OPR-AureFloFT-EN,
Rev H
Appendix H: Symbols & Signs
The following table contains a list of the possible symbols with accompanying denitions. Not all symbols
are applicable to all products. The term device may refer to any meter, monitor, probe, printer, uid
warmer or other Transonic
®
device.
FAILURE TO COMPLY WITH ALL WARNINGS BOTH WRITTEN AND SYMBOLIC COULD RESULT IN PATIENT
INJURY OR EQUIPMENT DAMAGE, AND VOID ANY AND ALL WARRANTIES.
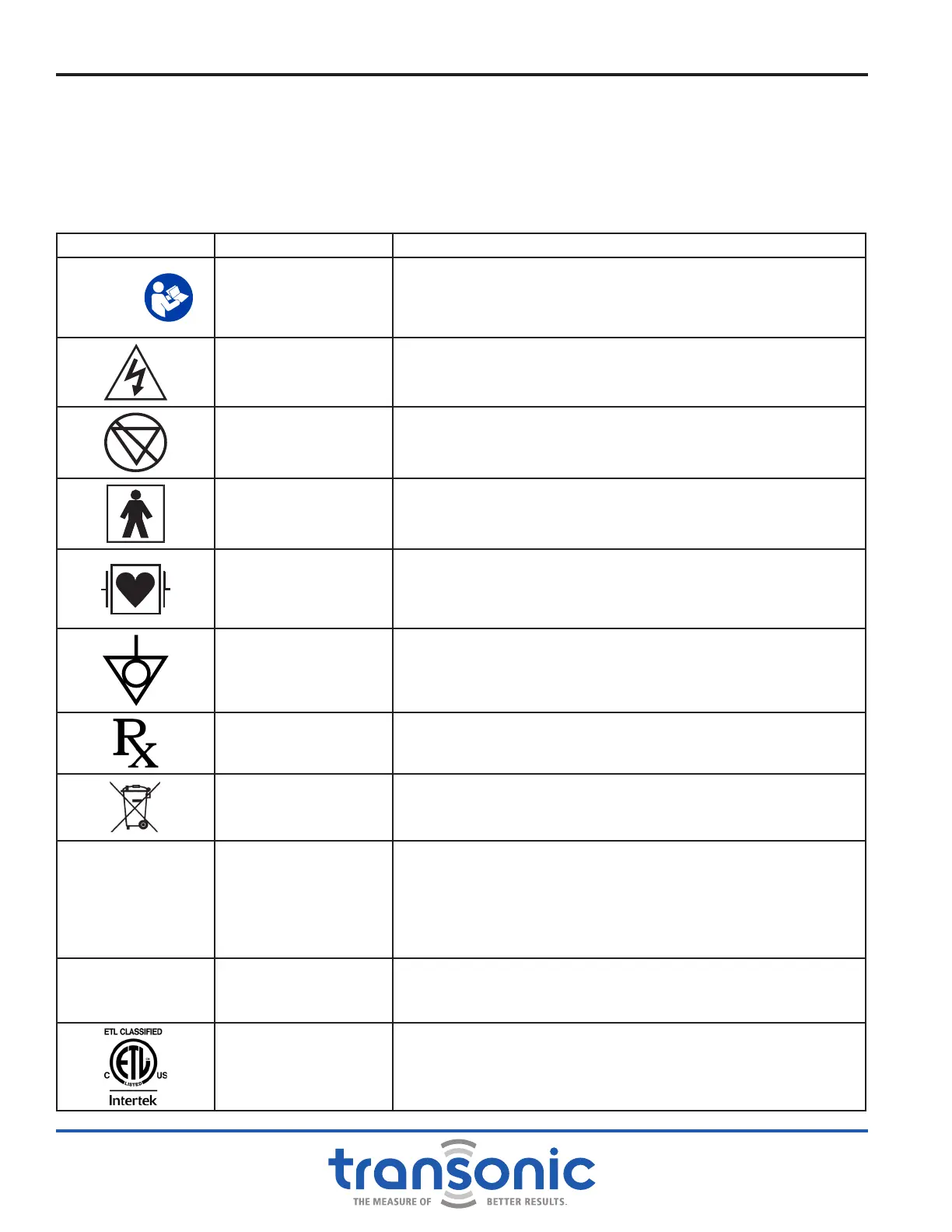
LEGEND SYMBOL DEFINITION TRANSONIC NOTATION
Y
Attention, Consult
Accompanying
Documents
The specic directions in this manual and in the package inserts
included with each device must be observed. Periodic testing
of devices must be performed to assure the validity of ow
measurements.
Dangerous Voltage
The device must not be modied or serviced except by qualied
Transonic
®
repair personnel.
AP
Not Category AP
Equipment
Danger-Explosion risk if used with ammable anesthetics.
Type BF Equipment
This device employs oating isolation to yield a high degree of
patient electrical protection in accordance with IEC 60601-1
Debrillator-Proof
Type CF equipment
This device employs line-to-meter, meter-to-probe and probe-to-
patient (cardiac oating) isolation to yield a high degree of patient
electrical protection when used with the proper cables.
Equipotentiality
This ground pin is connected to the metal cabinet of the monitor. It
provides the User with a means to equalize the electrical potential
when connecting the device to other equipment.
Prescription Device
Federal law (USA) restricts this device to sale or use by or on the
order of a physician.
Waste Electrical and
Electronic Equipment
This device contains material that requires special waste handling
procedures for disposal. Contact Transonic
®
Customer Service to
arrange for disposal.
D
Single Use Only: Do
Not Reuse
This device is to be disposed of after use on a single patient per
standard procedures for biohazardous materials. Reusing single-use
medical device or using it beyond the prescribed time may be unsafe.
Risks include, but are not limited to: cross-infection, contamination,
mechanical failure, patient injury or other associated patient health
risks.
C
2797
CE Conformity Mark
This device conforms to the requirements of applicable EU directives.
See the Declaration of Conformity accompanying this device for
specic directives.
ETL Testing Mark Electrical Safety Compliance Certication
 Loading...
Loading...