AU-OPR-AureFloFT-EN,
Rev H
1
I. Introduction
NOTE: In this manual, “Flowmeters” will refer to HT300 Series
(-FT) FlowTrace
®
Compatible Flowmeters. “Flowprobes” refer to
Perivascular Flowprobes. “Flowsensors” refer to Clamp-on Tubing
Flowsensors.
Transonic
®
AureFlo
®
System is a stand-alone, integrated system
used during surgery with Transonic
®
Perivascular Flowprobes
or with Transonic
®
Sterile Tubing Flowsensors to continuously
measure, display, capture and document absolute volume ow and
other derived parameters in vessels or tubing circuits (Fig. 1.1).
A. Intended Use
Transonic’s Optima Flowmeters, AureFlo System, and FlowXL
®
Flowmeters and Flowprobes and Flowsensors
use transit-time ultrasound technology to measure volume ow of blood and other liquids, and derived
parameters thereof, during surgical interventions and extracorporeal applications.
B. Indications for Use
Flow measurements are indicated to support surgeons and other clinical staff in identifying and evaluating
ow rates to support their clinical impressions. The Optima Flowmeters, AureFlo System, and FlowXL
®
Flowmeters and compatible perivascular Flowprobes are indicated for use in adult and pediatric patients
during surgeries on major and peripheral arteries, veins, and ducts; on non-aerated synthetic vessel grafts;
intraoperatively where surgery is medically indicated; at intraoperative sites which admit and retain
ultrasonic couplant; with minimal vessel manipulation or constriction, and where application does not
unnecessarily lengthen surgical procedure. The Optima Flowmeters, AureFlo
System, and FlowXL
®
Flowmeters and compatible tubing Flowsensors are
indicated for use in extracorporeal applications; on exible tubing specically
calibrated to the Flowsensor and in non-aerated media which are transparent to
ultrasound. No contraindications are identied for this product.
C. AureFlo
®
Monitor & FlowTrace
®
Software
The AureFlo
®
Monitor’s touch-panel display is loaded with Microsoft Windows
®
.
FlowTrace
®
Software works exclusively with HT300 (-FT) Series Compatible
Flowmeters to provide a real-time waveform display of volume ow, mean
ow, pulsatility index (PI), pressure and ECG. It is designed for use only with a
Transonic
®
safety-tested and approved touchscreen panel PC used in conjunction
with the AureFlo
®
System. During surgery FlowTrace
®
calculates the Diastolic/
Systolic (D/S) ratio of the bypass graft or diastolic fraction (DF) (see "FlowTrace
®
Settings" on page 16 for enabling DF).
FlowTrace
®
Software has four modes of operation. They are:
1) Realtime: Continuous display of ow (and ECG, if present) in real time, plus
calculated parameters
2) Recording: Captures the displayed ow data (and ECG, if present) for later
review and analysis
3) Snapshot: Captures the 8 seconds of ow data shown on the display for
printing or later review
4) Portfolio: Enables review of recordings and Snapshots with printing capability
to document ow information for the patient record
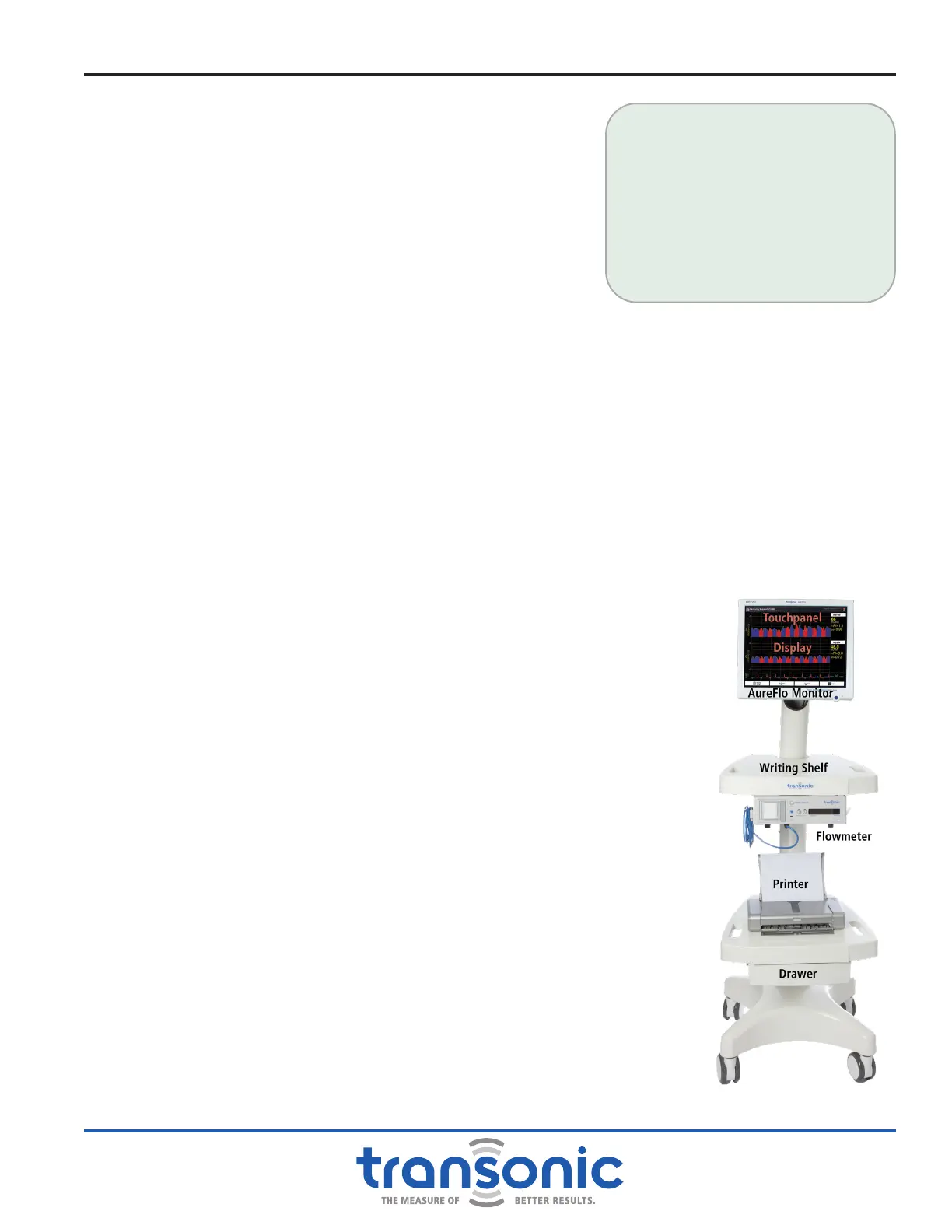
Fig. 1.1: AureFlo
®
System with Cart.
ECG SIGNAL
During surgery, an ECG signal can
be displayed on FlowTrace
®
at the
bottom of the touch-panel display.
The diastolic and systolic segments
of the waveform and the ECG will be
colorized. If an ECG is not connected
during surgery, the area under the
waveform will not be colorized.
 Loading...
Loading...