LOGIQ V2/LOGIQ V1 – User Guide i-1
Direction 5610736-100 Rev. 9
Revision History
Reason for Change
List of Effective Pages
Please verify that you are using the latest revision of this document. Information
pertaining to this document is maintained on MyWorkshop/ePDM (GE Electronic Product
Data Management). If you need to know the latest revision, contact your distributor, local
GE Sales Representative or in the USA call the GE Ultrasound Clinical Answer Center at
1 800 682 5327 or 1 262 524 5698.
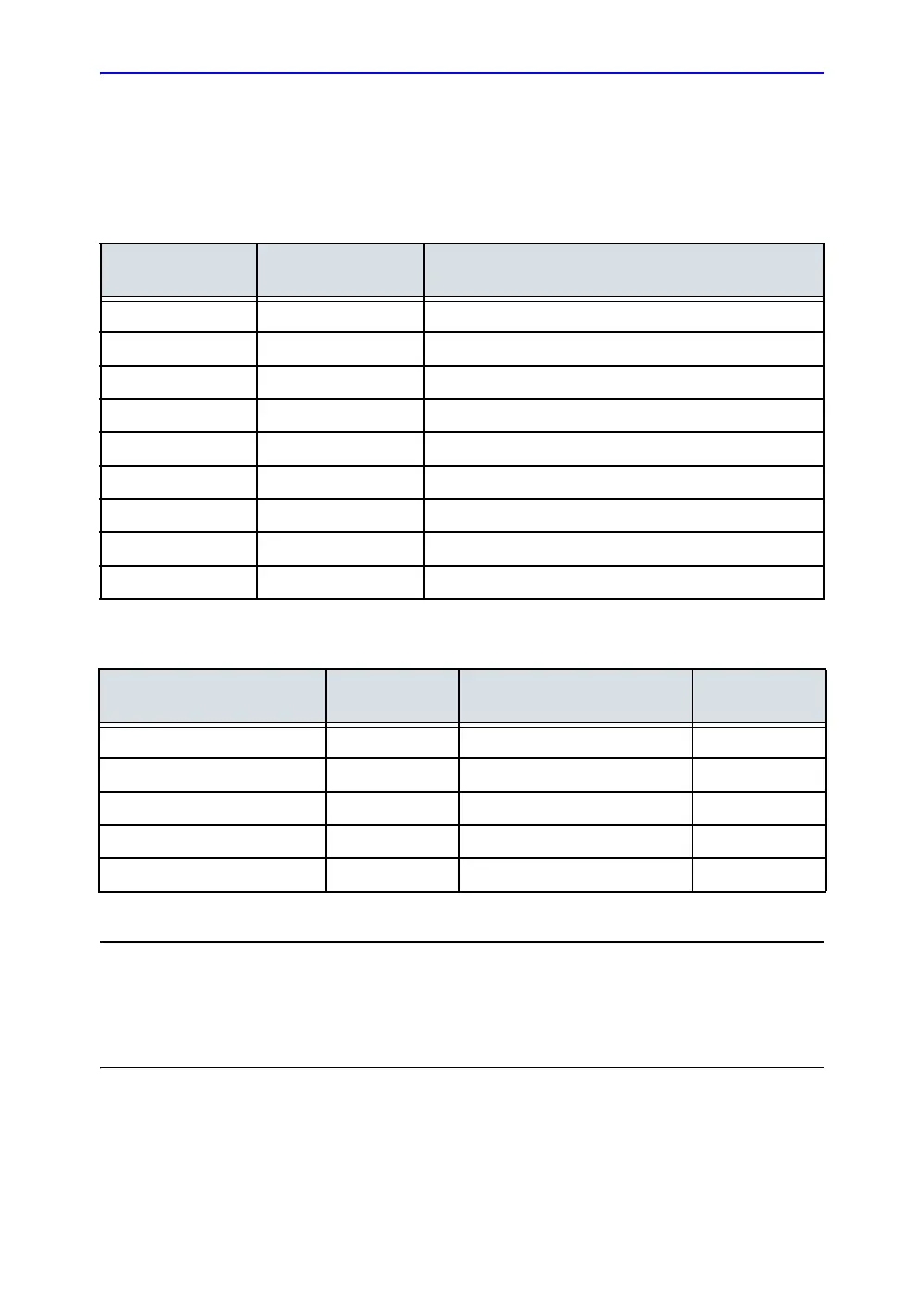
REV
DATE
(YYYY/MM/DD)
REASON FOR CHANGE
Rev. 1 2015/07/14 Initial release
Rev. 2 2015/10/14 Add onboard help
Rev. 3 2015/11/23 Remove secure wipe information
Rev. 4 2015/12/14 Update rating plate
Rev. 5 2016/02/25 Add intended use
Rev. 6 2016/06/14 Update rating plate
Rev. 7 2016/08/29 Add probe UDI label
Rev. 8 2016/12/08 Update software features
Rev. 9 2017/04/25 Update onboard help
CHAPTER NUMBER
REVISION
NUMBER
CHAPTER NUMBER
REVISION
NUMBER
Title Page Rev. 9 Chapter 3 Rev. 9
Revision History Rev. 9 Chapter 4 Rev. 9
Regulatory Requirements Rev. 9 Chapter 5 Rev. 9
Chapter 1 Rev. 9 Index Rev. 9
Chapter 2 Rev. 9
 Loading...
Loading...