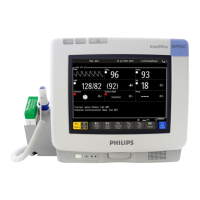
Preparing to Install the Monitor
68
Installin
the Instrument
The following are the markings on the back of the Monitor:
The printer port uses LED devices for infrared communication with the printer. These LED
devices are measured to be AEL Class 1 LED Products per IEC 825-1 and CENELEC
EN60825-1 Standards.
The Philips M3046A Compact Portable Patient Monitor complies with the
requirements of the Council Directive 93/42/EEC of 14 June 1993
(Medical Device Directive) and Council Directive 1999/5/EC of 9 March
1999 (Radio and Telecommunications Terminal Equipment Directive).
This device complies
with FCC part 15
of the FCC rules.
Operation is subject to
the following two conditions:
(1) this device may not
cause harmful interference.
and
(2) this device must accept
any interference received,
including interference that
may cause undesired
operation.
Prod No. M3046A Opt.
SN: XXXXXXXXXX
D-71034 Boeblingen,
Germany
CLASS 1
LASER
Made in
Germany
0366
Philips
S
NRTL/C
R
PRODUCT
2003-05
0560
Radio module inside
FCC ID IMKRL2630M
0366
0560
 Loading...
Loading...