Model 3430 Plus & 3440 Plus
RADIOACTIVITY
Radioactivity is the spontaneous transformation (or disintegration)
of an unstable nucleus into a more stable configuration accompanied
by the emission of radiation.
The quantity of a radioactive material is measured in terms of the
average number of nuclear disintegrations per unit time. The
traditional unit of measure for radioactivity (or activity) is the curie
(Ci), which is defined as 3.7 × 10
10
disintegrations per second. The
activities of the radioactive sources in nuclear gauges are so small
that they are typically measured in millicuries (mCi), which is one-
thousandth of a curie, or microcuries (µCi), which is one-millionth
of a curie.
In the Standard International (SI) (or metric) system, the unit of
activity is the becquerel (Bq), which equals one disintegration per
second. Because the becquerel is such an extremely small unit, the
activity of sources in nuclear gauges is normally expressed in
megabecquerel (MBq), which is one million becquerels, or
gigabecquerel (GBq), which is one billion Bq.
The radioactivity of a source is not constant, but decreases with time
as the source decays. The time it takes for one-half of the original
atoms to disintegrate is called the half-life. In successive half-lives,
the activity decreases to 1/2, 1/4, 1/8 and so on of the initial value.
After seven half-lives, less than 1% of the original radioactive atoms
remain. Each radioisotope has a characteristic half-life, which can
range from seconds to billions of years. The half-lives for the
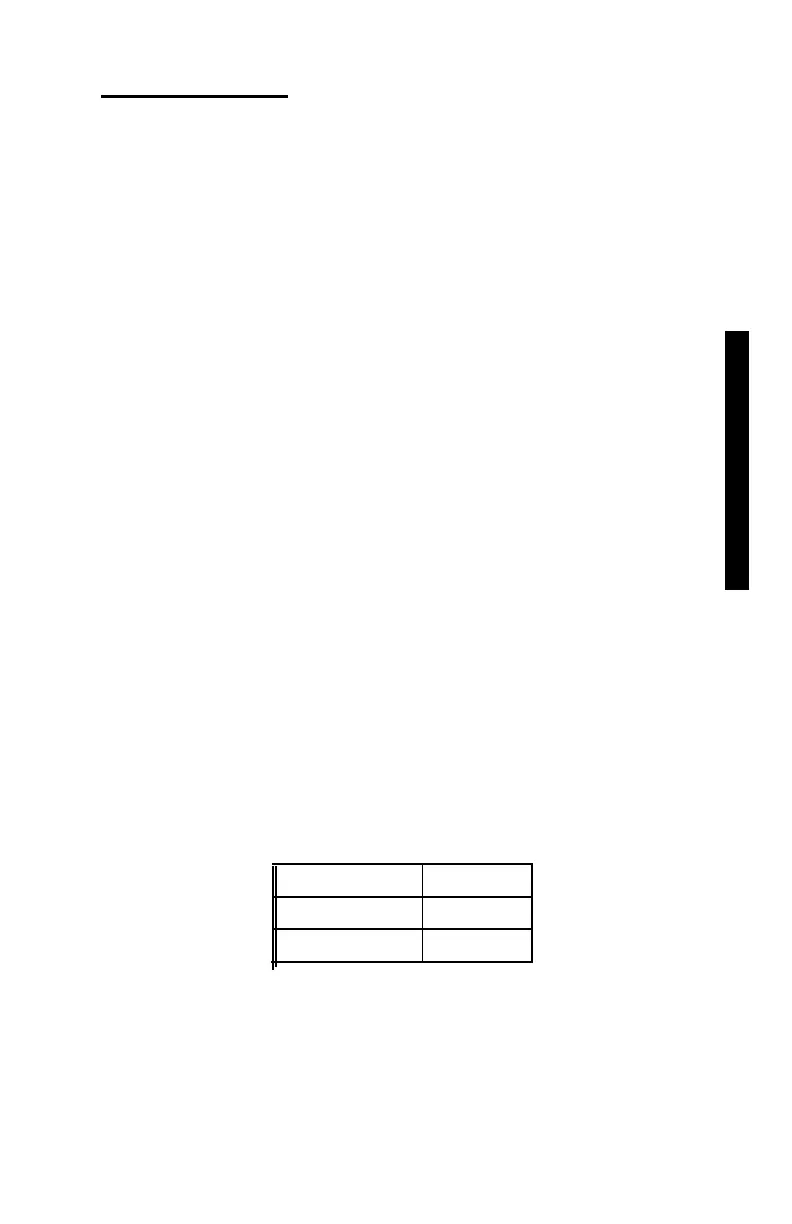
typical radioisotopes used in nuclear gauges are:
Cs-137 30 years
Am-241 432 years
 Loading...
Loading...











