4 - 11
Transpector MPH Operating Manual
Ionization probability factors can be approximated by substituting the relative ion
gauge sensitivities for various gases. Table 4-5 gives relative ion gauge
sensitivities for some common gases.
NOTE: The data was compiled from Empirical Observations on the Sensitivity of
Hot Cathode Ionization Type Vacuum Gauges by R. L. Summers (NASA
Technical Note NASA TN D5285, published in 1969). Similar, although
more limited, lists of ionization sensitivities can be found in the books by
O’Hanlon (Chapter 8, Section 1.1) and Drinkwine and Lichtman (Table I,
page 5).
HINT: Actual ionization probabilities vary significantly depending on the ionizer
and the electron energy. For best accuracy, measure the relative ionization
probability using a hot cathode ionization gauge (calibrated for nitrogen) to
monitor a known pressure of the substance of interest. The ratio of the
gauge reading to the known true pressure is the relative ionization
probability. To determine the true pressure, use a gauge which is gas
species independent (for example, a capacitance manometer) or a gauge
with a known sensitivity factor (for example, a spinning rotor gauge).
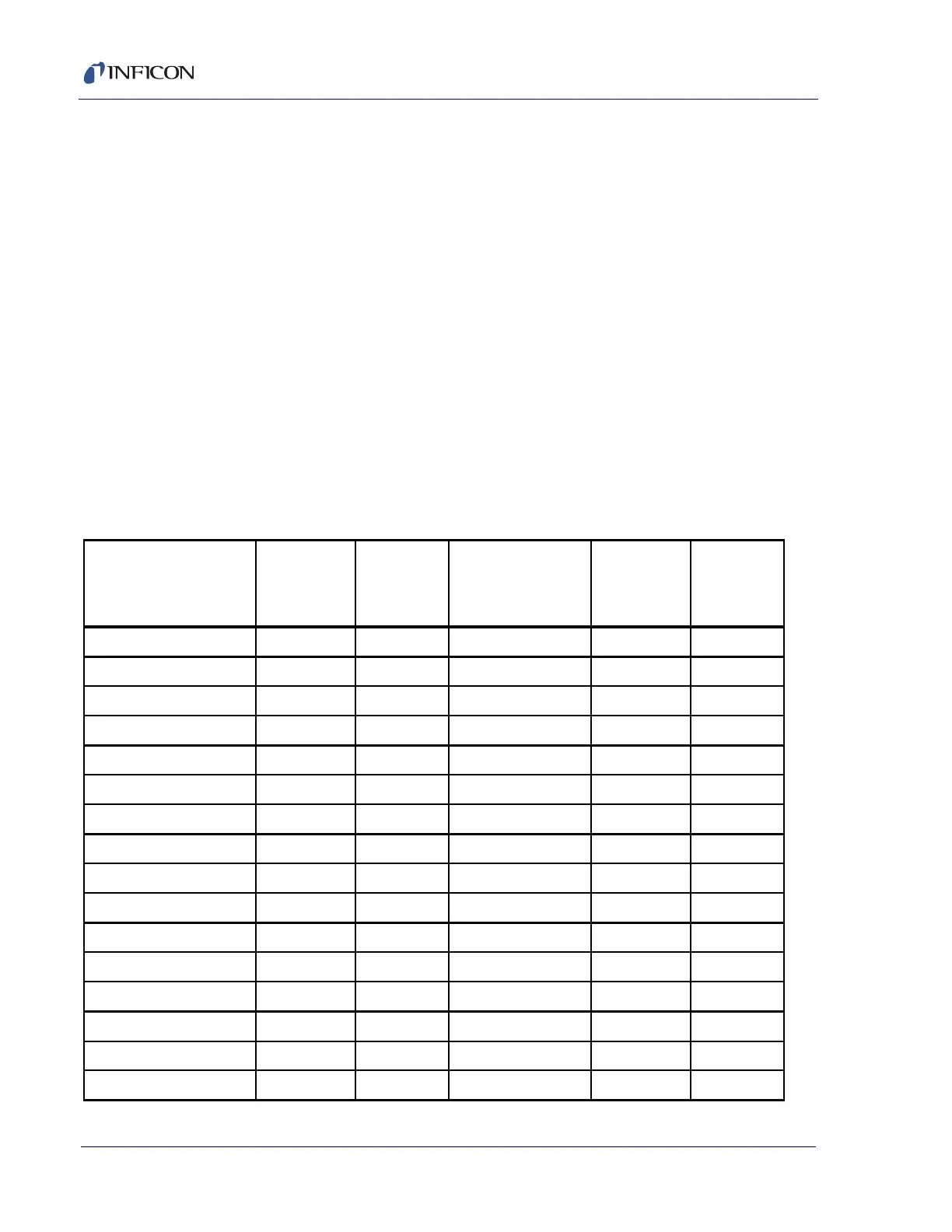
Table 4-5 Ionization Probabilities for some Common Substances
Substance Formula
Relative
Ionization
Gauge
Sensitivity
Substance Formula
Relative
Ionization
Gauge
Sensitivity
acetone (CH
3
)
2
CO 3.6 hydrogen chloride HCl 1.6
air 1.0 hydrogen fluoride HF 1.4
ammonia NH
3
1.3 hydrogen iodide HI 3.1
argon Ar 1.2 hydrogen sulfide H
2
S2.2
benzene C
6
H
6
5.9 krypton Kr 1.7
benzoic acid C
6
H
5
COOH 5.5 lithium Li 1.9
bromine Br
2
3.8 methane CH
4
1.6
butane C
4
H
10
4.9 methanol CH
3
OH 1.8
carbon dioxide CO
2
1.4 neon Ne 0.23
carbon disulfide CS
2
4.8 nitrogen N
2
1.0
carbon monoxide CO 1.05 nitric oxide NO 1.2
carbon tetrachloride CCl
4
6.0 nitrous oxide N
2
O1.7
chlorobenzene C
6
H
5
Cl 7.0 oxygen O
2
1.0
chloroethane C
2
H
5
Cl 4.0 n-pentane C
5
H
12
6.0
chloroform CHCl
3
4.8 phenol C
6
H
5
OH 6.2
chloromethane CH
3
Cl 3.1 phosphine PH
3
2.6
 Loading...
Loading...