Rev. 2 41
Calibration
Calibration methods
Note
All references to “dielectric constant” herein refer to the unitless
relative dielectric to
0
(
0
is the dielectric constant of a vacuum).
4.2.4 Capacitance-based Sensor Calibration Methods
The most straightforward calibration method is the Open Dewar
Calibration which requires the customer to have access to a filled dewar
where the full active length of the sensor can be dipped. The Closed Dewar
Calibration method can be performed in situations where it is not feasible
for the customer to dip the sensor into an open dewar, such as situations
where the target liquid is under pressure. The closed dewar calibration is
more complex and may require initial preparations to insure success.
Occasionally customers ask AMI to calibrate an instrument and sensor for
a liquid which is not available at AMI for calibration purposes and/or for a
sensor which is too long to be calibrated at our facilities.
For the case of the target liquid being unavailable, AMI uses liquid
nitrogen as the reference liquid and an Approximate Calibration is
performed using mathematical manipulation of the ratio of the dielectric
constants between liquid nitrogen and the desired liquid. This procedure is
outlined in the Approximate Calibration section beginning on page 49. The
technique is intended to provide the instrument with an approximate
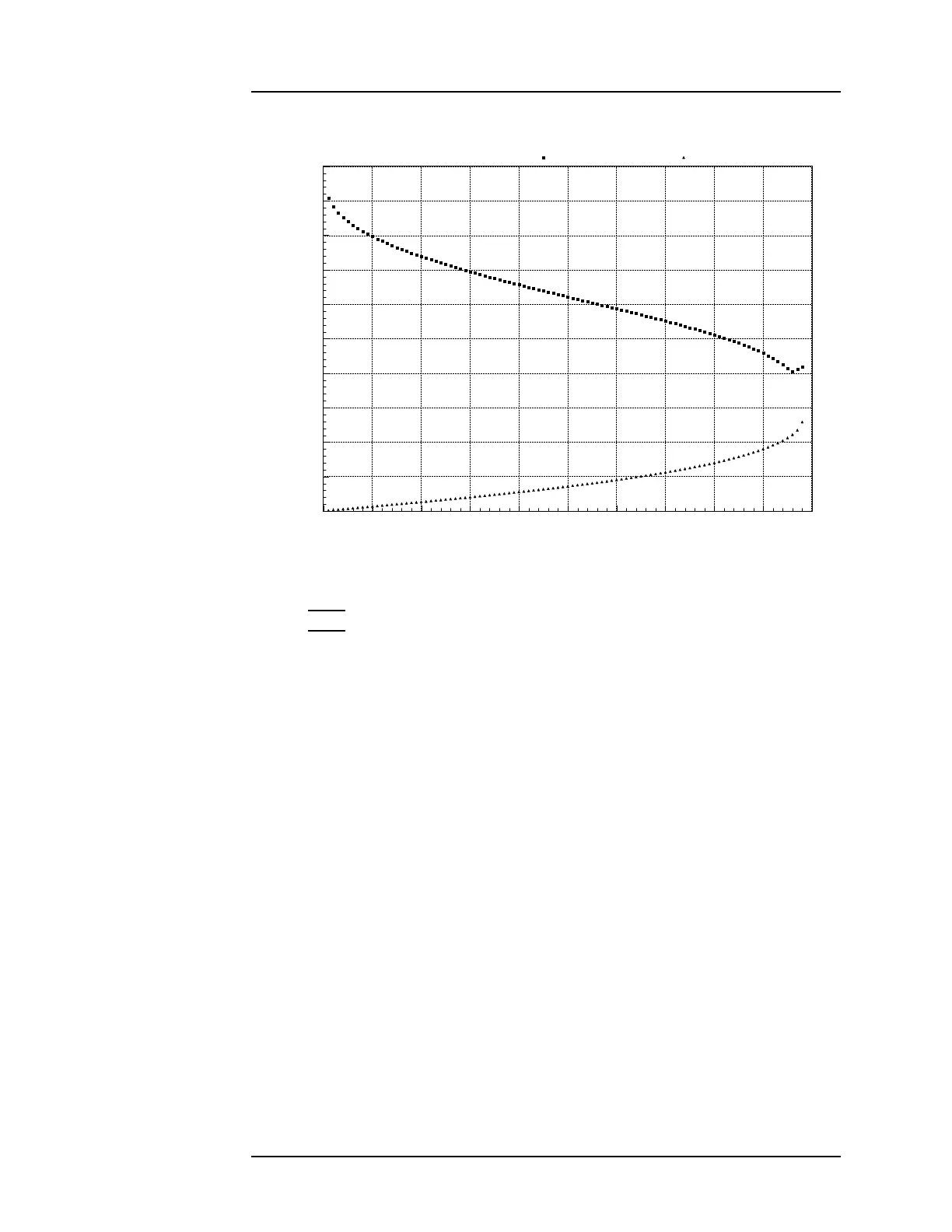
0 50 100 150 200 250 300 350 400 450 500
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
Diel-1 (saturated liquid) Diel-1 (saturated vapor)
Pressure (psi)
Diel - 1
Figure 4-6. Dielectric vs. pressure for nitrogen under saturated conditions.

 Loading...
Loading...