Affirm Breast Biopsy Guidance System Service Manual
Appendix D: Biopsy Device Evaluation Procedure
Page 84 MAN-05754 Revision 002
Configure a New Biopsy Needle
Obtain Needle Specifications
1. Record the following technical information from the biopsy device manufacturer:
Needle Name ______________________________
Aperture ______________________________mm
Dead Space ______________________________mm
Diameter (convert the needle
gauge listed by the manufacturer
into millimeters)
______________________________mm
Length ______________________________mm
Pre-Fire Pullback (calculate by
adding dead space + 1/2 aperture,
then subtract the stroke)
______________________________mm
Stroke ______________________________mm
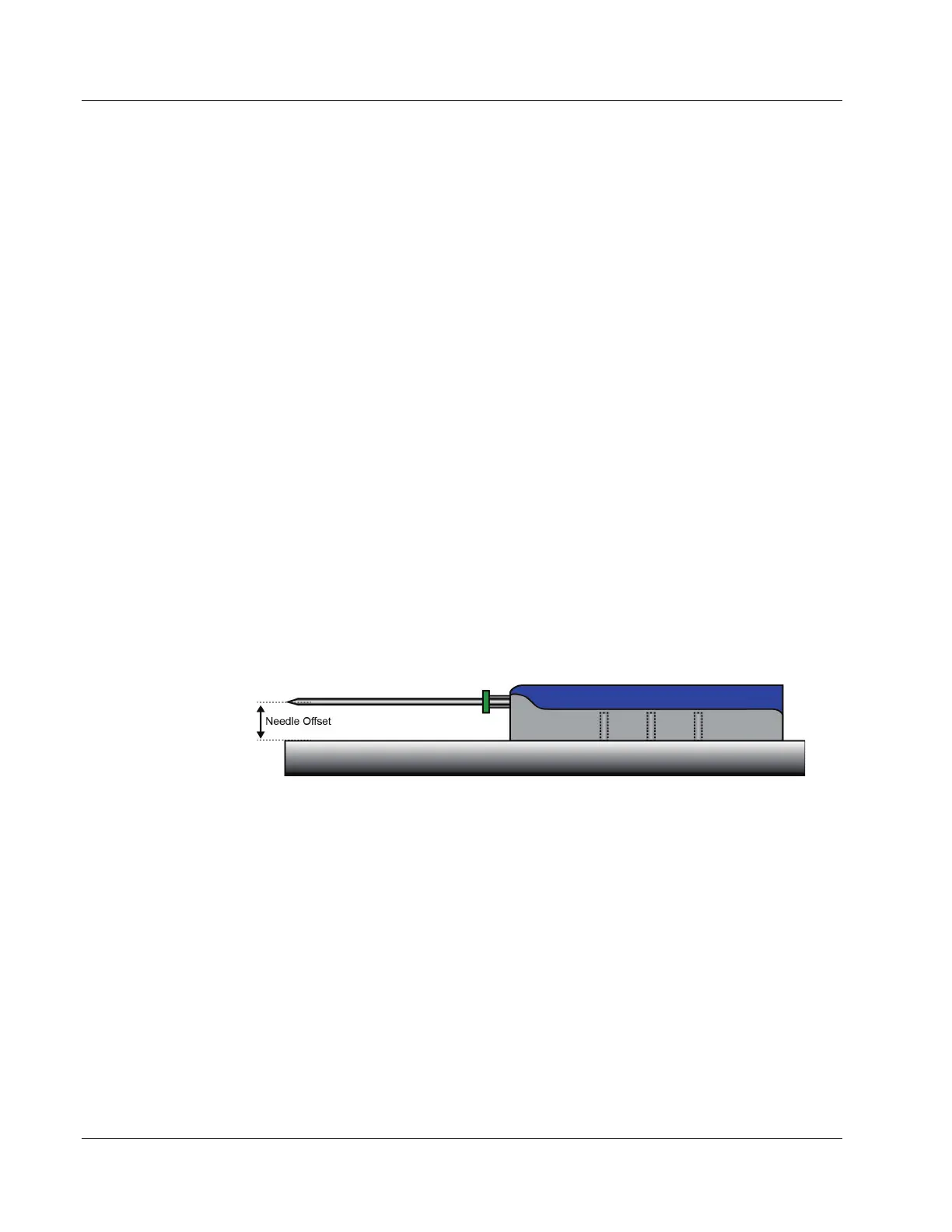
2. Record the needle offset measurement with the device in the extended (or post-fire)
position. Unless otherwise specified by the manufacturer, use 37.5 mm.
The needle offset is the vertical distance between the center of the needle and bottom
of the device holder. See the following figure.
Needle Offset (NO) =_____________mm.
Figure 53: Needle Offset with Biopsy Device in Extended (post-fired) Position
 Loading...
Loading...