E-2
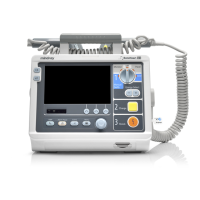
E.2 Device Enclosure and Accessories
E.2.1 Visual Inspection
Test Item Acceptance Criteria
The enclosure and accessories
No physical damage to the enclosure and
accessories.
No physical damage to meters, switches,
connectors, etc.
No residue of fluid spillage (e.g., water, coffee,
chemicals, etc.).
No loose or missing parts (e.g., knobs, dials,
terminals, etc.).
E.2.2 Contextual Inspection
Test Item Acceptance Criteria
The enclosure and accessories
No unusual noises (e.g., a rattle inside the case).
No unusual smells (e.g., burning or smoky
smells, particularly from ventilation holes).
No taped notes that may suggest device
deficiencies or operator concerns.
E.3 Device Labelling
Check the labels provided by the manufacturer or the healthcare facilities are present and
legible.
Main unit label
Integrated warning labels
 Loading...
Loading...