10
Roche Diagnostics
cobas
®
pulse · Software version 01.03 · User Assistance · Publication version 1.0
Symbol Explanation
Materials that are required for a task
Prerequisites of a task
y Symbols used in the publication
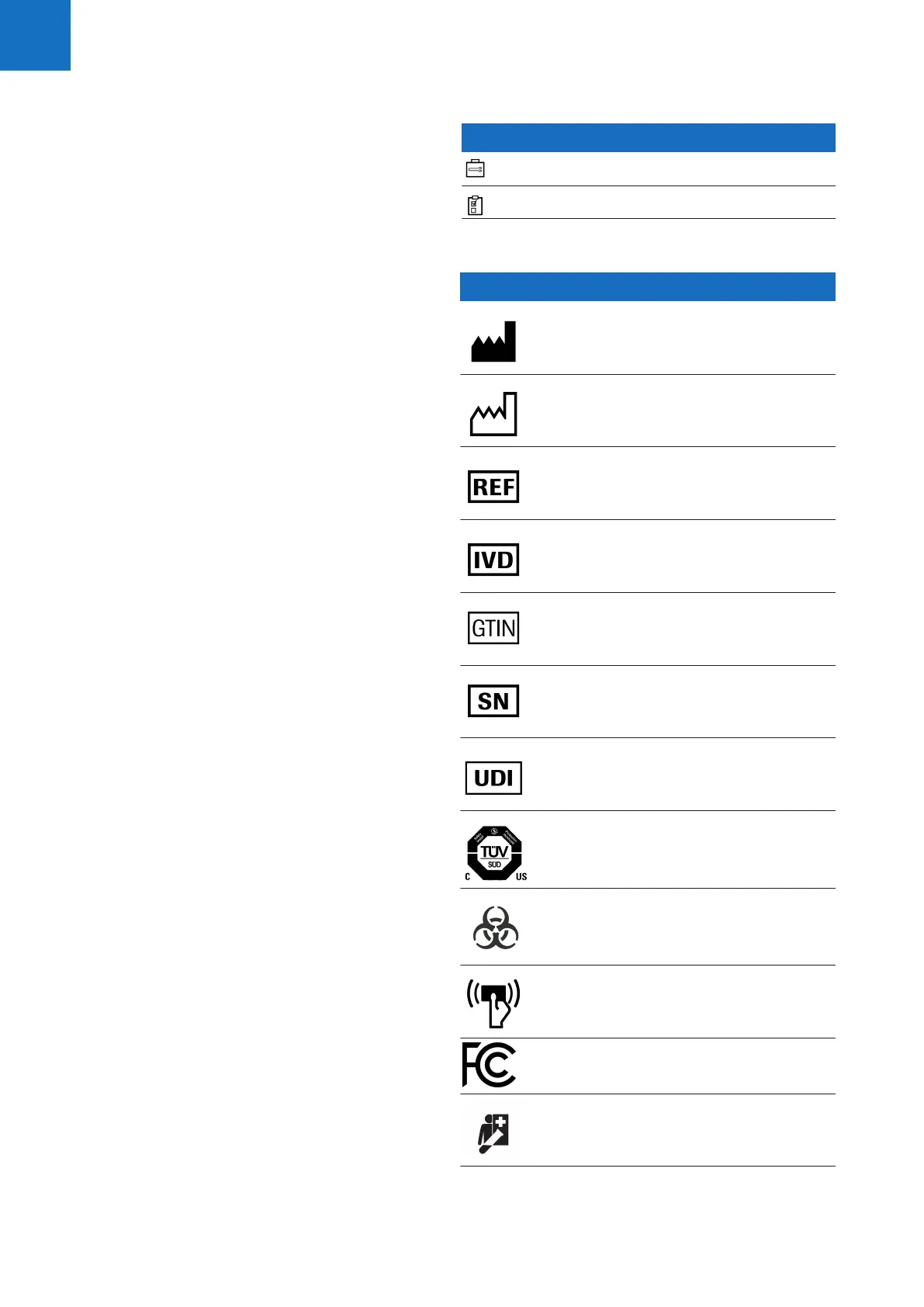
Symbols used on product and packaging
Symbol Explanation
Manufacturer.
Date of manufacture.
Catalog number.
In vitro diagnostic medical device.
Global Trade Item Number.
Serial number.
Unique device identifier
TÜV SÜD U7-2D
Biohazardous
RFID reader.
Device contains a transmitter.
Device for near-patient testing
y Symbols used on product and packaging
 Loading...
Loading...