16 BioPAT
®
Trace | Multi Trace Operating Instructions
BioPAT
®
Trace | Multi Trace Product Description
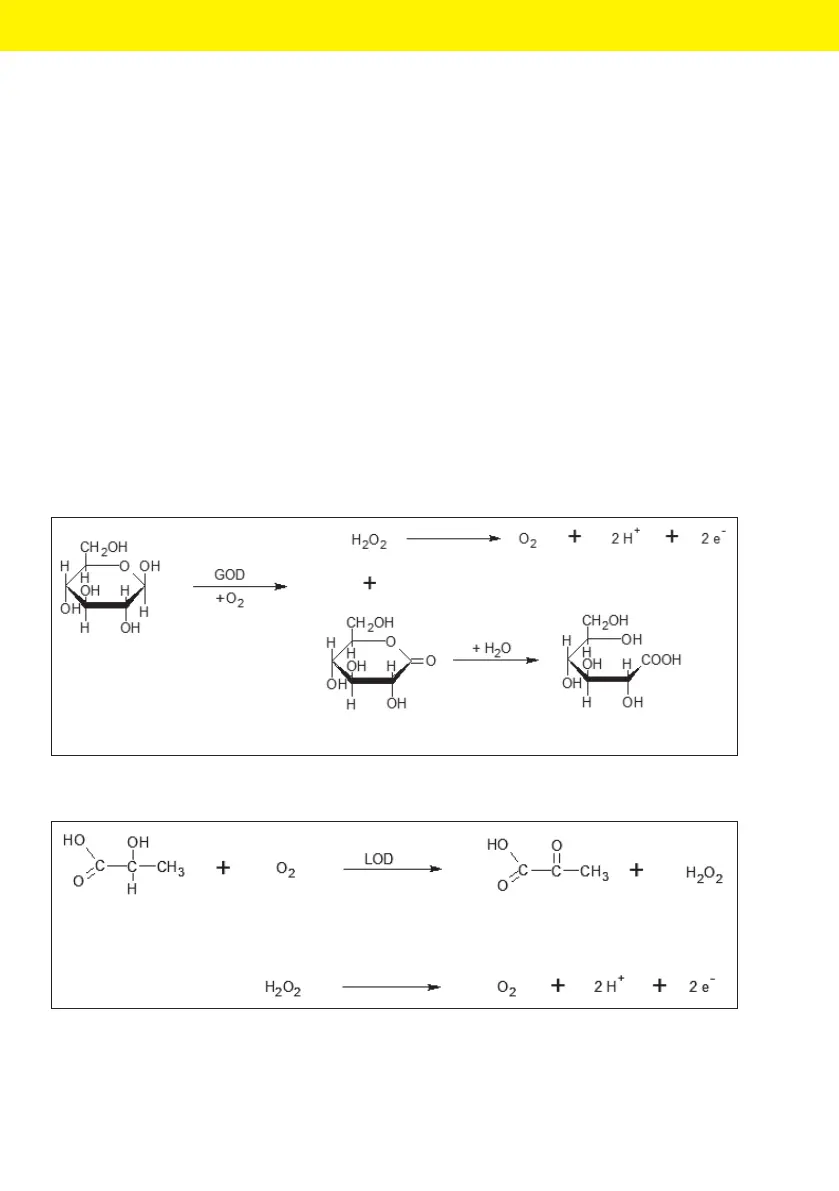
3.2 Biosensor Principle
The analytes glucose and lactate are detected by means of enzymatic reac-
tions. Figure 1 shows the enzymatic conversion of glucose to gluconolactone.
In the presence of water, gluconolactone is immediately hydrolyzed to glu-
conic acid. The hydrogen peroxide (H
2
O
2
) produced in the first step is
detected amperometrically through anodic oxidation that releases the two
electrons. Figure 2 shows the enzymatic conversion of lactate to pyruvate.
During this reaction as well, H
2
O
2
is formed and then detected
amperometrically.
Enzyme reactions take place according to the “key-lock principle”. They are
specific and thus highly selective. Therefore, the reaction at the enzyme sys-
tem is a further selection. The combination of the two selective principles
makes the BioPAT
®
Trace | Multi Trace largely insensitive to matrix effects and
extraneous materials.
β-D-Glucose
D-Glucono-δ-lacton D-Gluconic acid
Fig. 1: Enzymatic conversion of glucose with parallel anodic oxidation of H
2
O
2
Lactate Pyruvate
Fig. 2: Enzymatic conversion of lactate with parallel anodic oxidation of H
2
O
2
 Loading...
Loading...