Review Results
159
Quality control (QC) results
This memory area contains all test parameter-specific quality control tests (QC, starting on
page 144) that were run, sorted chronologically. The most recent results are at the top of the list.
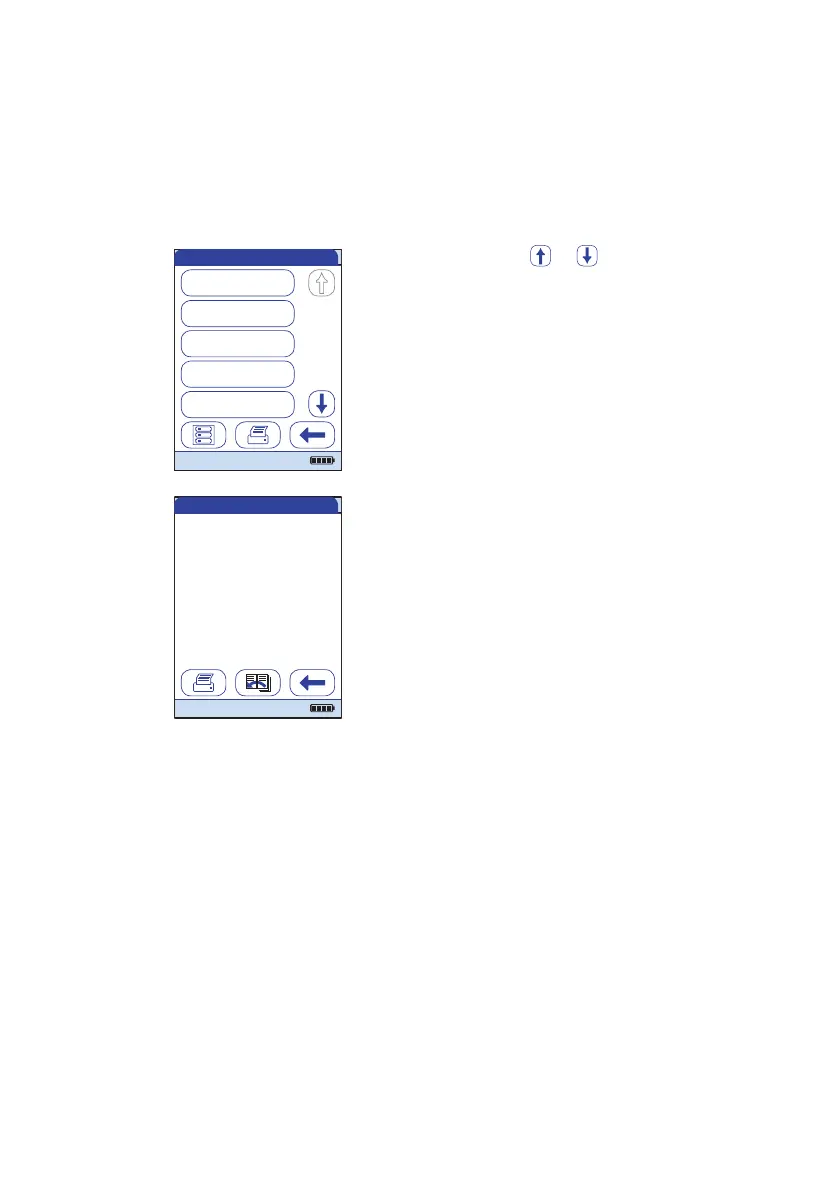
1 Touch or to display the entry of
choice on the screen.
2 Touch the entry you want to open.
The entry is displayed.
QC Results
DDimer L1
02/12/16 08:00
DDimer L2
02/12/16 08:30
proBNP L1
02/04/16 10:17
DDimer L2
01/10/16 07:30
DDimer High
01/10/16 07:05
04/19/2016
QC Results
329
02/04/2016 10:17
pg/mL
(350 / 300-400 pg/mL)
Level:
proBNP PB1234
PBC014
JONES, TOM
Par:
Op:
Code:
QC: 1
Pass:
04/19/2016
 Loading...
Loading...