Review Results
160
Instrument quality control (IQC) results
This memory area contains all instrument quality control tests (IQC, starting on page 152) that
were run, sorted chronologically. The most recent results are at the top of the list.
1 Touch or to display the entry of
choice on the screen.
2 Touch the entry you want to open.
The entry is displayed.
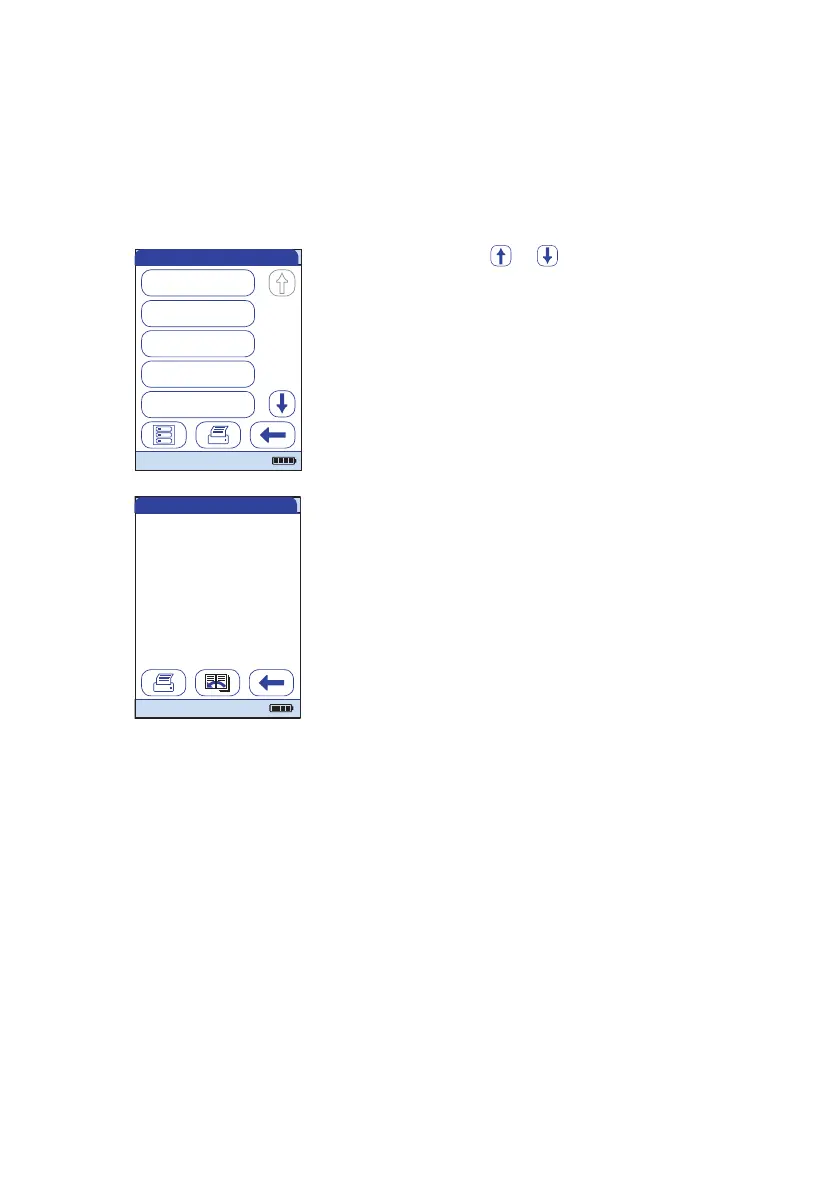
IQC Results
IQC Pass
04/11/16 07:59
IQC Pass
04/07/16 08:16
IQC Fail
04/07/16 08:01
IQC Pass
03/29/16 08:30
IQC Pass
03/29/16 09:02
04/19/2016
QC Test - Result
IQC Code:
JONES, TOM
Par:
Op:
04/19/2016 07:59
Level: 1
IQC702
83
%
(85 / 82 - 88 %)
04/19/2016
 Loading...
Loading...