H50/H51 Hematology Analyzer User Manual QC
- 26 -
Chapter 8 QC
To control the stability of the instrument before running patient samples, it is mandatory to run
QC test at the beginning of the working day. If the QC test results are out of control, it is
recommended to perform a calibration, then run QC test again.
NOTE:
Take safety measures when working with biological samples, such as wearing
approved gloves, etc.
Only controls provided by EDAN or its authorized distributors can be used. If not, Edan
is not responsible for any adverse consequences such as test results’ accuracy,
analyzer’s troubleshooting, maintenance and repair, etc.
Press QC to enter into Screen 7-1. Press Changes to enter into Screen 7-2. Press Results to enter
into Screen 7-3.
Screen 7-1
Screen 7-2
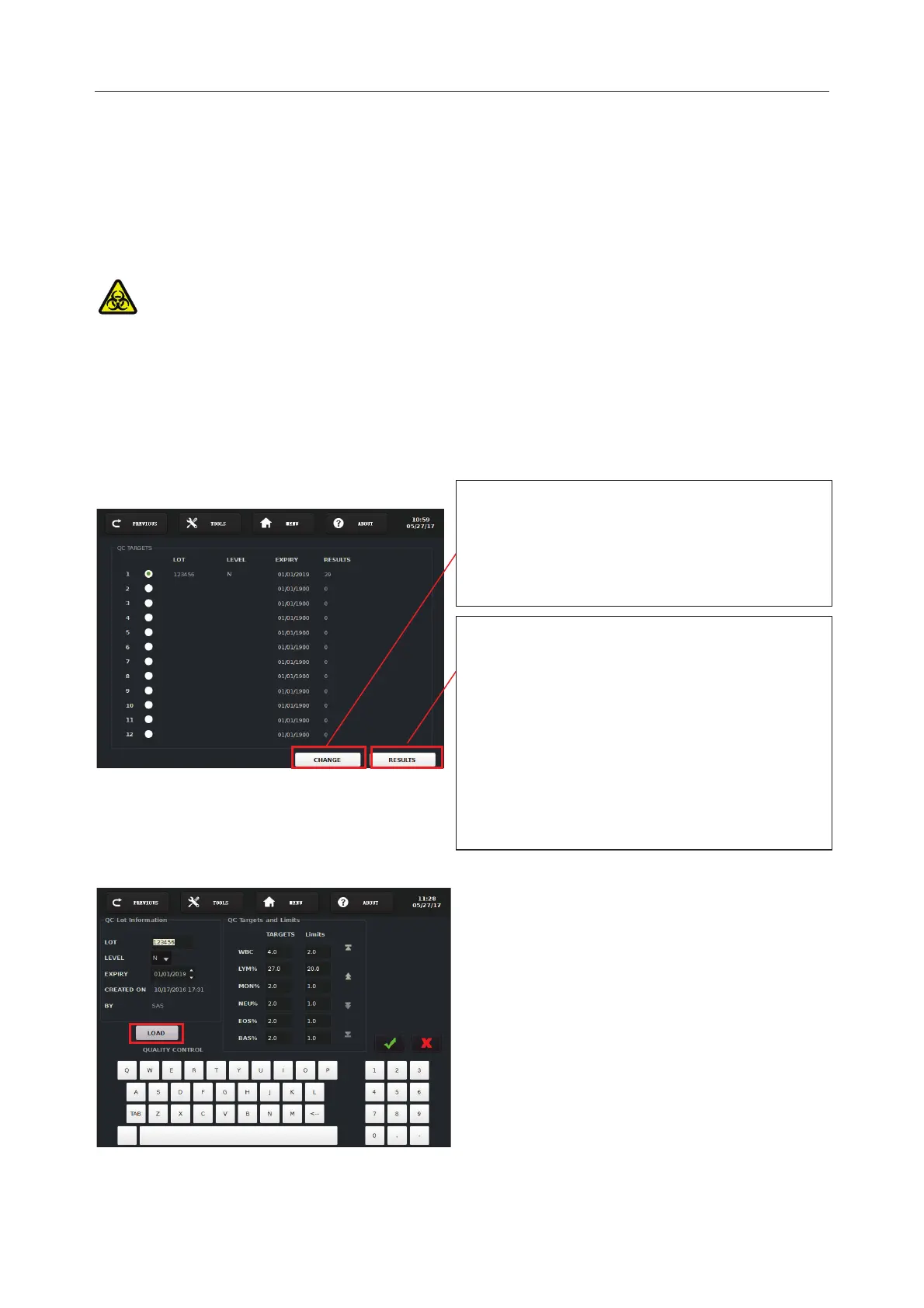
“CHANGE” button allows creating a new QC
lot
or re-editing the existent QC
all its ass
ociated results will be deleted if
you re
-edit the existent QC information.
You can directly go to “RESULTS” to do QC
test or view
QC test results. T
he sampling
needle will move
down
to the sampling
position automatically. Present controls under
the sampl
ing needle and press the trigger
, the
analyzer will automatically do the sample
aspiration and analysis, and finally display the
test results on the screen.
The Screen 7-2 is mainly used to edit control
material
’s information.
Lot, Level, Expiry,
Limits on the Screen 7-2:
Enter manually by Alpha-
screen keyboard
Import the information from USB drive
with the button “LOAD”

 Loading...
Loading...