16.1 China
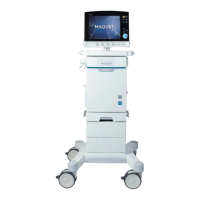
16.1.1 FLOW-i
SFDA(I)20123542189CFDA registration no
YZB/SWE 2546-2012Product standard no
For manufacturing date, see label on the deviceManufacturing date
Maquet Critical Care ABManufacturer
Rontgenvagen 2, SE-17154 Solna, SwedenManufacturer/Manufacturer site address
Maquet (Shanghai) Medical Equipment Co., Ltd.Agent for registration and after sales
Room 227, 2nd floor, No. 56, Meisheng Road, Pilot
Free Trade Zone, Shanghai, China
Agent address
800 820 0207Agent contacts
150904IFU revise date
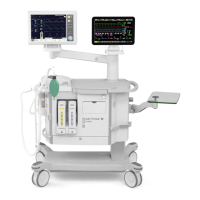
16.1.2 CO
2
absorber
CFDA(I)20131542673CFDA registration no
YZB/SWE 2969-2013Product standard no
For manufacturing date, see label on the deviceManufacturing date
Maquet Critical Care ABManufacturer
Rontgenvagen 2, SE-17154 Solna, SwedenManufacturer/Manufacturer site address
Maquet (Shanghai) Medical Equipment Co., Ltd.Agent for registration and after sales
Room 227, 2nd floor, No. 56, Meisheng Road, Pilot
Free Trade Zone, Shanghai, China
Agent address
800 820 0207Agent contacts
150904IFU revise date
302
FLOW-i 4.2, User's Manual
| 16
| Certificates |
 Loading...
Loading...