Do you have a question about the Getinge Maquet FLOW-i 4.2 and is the answer not in the manual?
Specifies the clinical situations for which the FLOW-i Anesthesia System is intended.
Details the intended purpose and user groups for the anesthesia system.
Provides guidance on how to navigate and understand the structure of the user manual.
Provides key information and definitions applicable throughout the user manual.
Details the necessary steps and precautions for connecting the anesthesia system.
Covers essential operational guidelines, safety measures, and system capabilities.
Outlines requirements for system installation and maintenance procedures.
Specifies requirements and considerations for connecting external equipment.
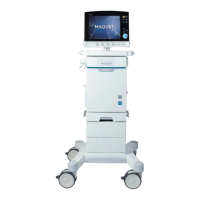
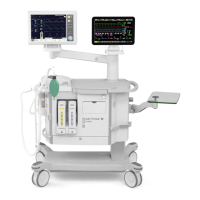
Identifies and illustrates the main components of the anesthesia system.
Describes the components and layout of the system's primary control interface.
Details the requirements and compatibility for connecting an optional patient monitor.
Identifies the components that constitute the anesthesia system's breathing circuit.
Describes the unit that holds and manages anesthetic vaporizers.
Explains the system's capability for manual ventilation during power or system failures.
Illustrates and lists the various external ports for power and gas connections.
Provides definitions and meanings for symbols and labels found on the system.
Details how to position the anesthesia system for optimal operator ergonomics.
Provides guidelines for safely storing and transporting the anesthesia system.
Describes the different available models of the anesthesia system and their features.
Lists and describes various optional accessories that can be attached to the system.
Instructions on how to initiate the system's power-on sequence.
Details the mandatory procedures for ensuring system functionality and patient safety.
Describes the process for verifying the integrity of breathing circuits against leakage.
Outlines the procedure for checking vaporizer functionality and preventing agent leaks.
Explains how to interpret the outcomes of system checkout procedures.
Describes the system's state when idle, including available functions.
Procedure for initiating a new patient case after system checkout.
Details how to check and operate the emergency ventilation system.
Overview of the FLOW-i system's design, components, and operational principles.
Explains how to input and utilize patient data for ventilatory parameter recommendations.
Details how to configure and manage the fresh gas supply mixture and flow rates.
Describes available manual and automatic ventilation modes and their configurations.
Lists the various ventilation and gas parameters that can be displayed and monitored.
Explains the customization and display options for real-time waveforms and trends.
Describes how to use hold functions to estimate lung characteristics like compliance.
Covers system settings such as date/time, power status, and timer functions.
Provides an overview of the system's breathing circuit components.
Details the patient cassette as the central hub for gas flow and its connections.
Explains the function and operation of the manual oxygen flush mechanism.
Describes the switch used to change between manual and automatic ventilation modes.
Details the Adjustable Pressure Limit valve and its mechanical regulation.
Explains the volume reflector's function as a reservoir for exhaled gases.
Describes the AGS system, its flowmeter, and connection requirements.
Recommends specific lengths and types of patient tubing for optimal system performance.
Details the manual breathing bag and its connection to the patient cassette.
Explains the function of the water trap and sampling line for gas analysis.
Describes the CO2 absorber, its capacity, and switch positions.
Details the operation and testing of the optional auxiliary oxygen and suction module.
Explains the operation of the built-in emergency ventilation system.
Lists compatible anesthetic agents and describes the LED status indicators.
Describes the gas analyzer's technology, measurements, and accuracy specifications.
Details the operation, duty cycle, and capacity of the lift function for the C30 model.
Explains the attachment, installation, and safety considerations for the C40 model's ceiling pendant.
Introduces the AFGO option and its function in bypassing the circle system.
Lists the requirements and steps necessary before using the AFGO outlet.
Describes how to select and configure AFGO ventilation modes.
Provides additional details on AFGO usage, including inaccessible functions and manual maneuvers.
Provides an overview of the membrane buttons and their functions.
Explains the function of the audio pause button for muting alarms.
Describes how to view and configure applicable alarms and their limits.
Details the options for starting a new patient case and completing an ongoing one.
Explains how to save a copy of the current screen to a USB memory device.
Describes how to view trend data in numerical or graphical format.
Explains the operation of the system's timer function using membrane buttons.
Describes how the Home button cancels current settings and returns to the main screen.
Allows user configuration of screen layout, brightness, and waveform display.
Provides access to system functions like patient settings, checkout, and service.
Overview of system alarms, their visual and auditory indicators, and priority levels.
Guides the user on how to specify and adjust alarm limits for various parameters.
Explains the functionality of muting alarms temporarily or until condition resolves.
Lists and describes high, medium, low priority clinical and technical alarm messages.
Details the system's safety features, including pressure relief and built-in safety valves.
Describes unique and inactivated alarms specific to the AFGO function.
Covers system power status, battery backup, and behavior during mains power failure.
Details system behavior during loss of central gas pressure and backup gas supply options.
Introduces the chapter's content on ventilation modes, settings, and safety.
Describes manual ventilation using a breathing bag and its important considerations.
Details AFGO ventilation, its open system nature, and important considerations.
Overview of automatic ventilation types: controlled, supported, and combined modes.
Explains Pressure Control ventilation, its parameters, properties, and expiration criteria.
Describes Volume Control ventilation, parameters, properties, and important considerations.
Details Pressure Regulated Volume Control, its properties, and important considerations.
Explains Pressure Support ventilation, parameters, properties, and important considerations.
Describes SIMV modes, mandatory breaths, and spontaneous breathing support.
Provides instructions on how to properly switch the system off and manage battery charging.
Provides general recommendations and cautions regarding cleaning procedures.
Discusses hygiene practices, bacterial/viral filter use, and water quality for cleaning.
Summarizes cleaning, disinfection, and autoclaving procedures with and without filters.
Details the steps for preparing the system and dismantling its components for cleaning.
Provides instructions for wiping down surfaces and discarding disposable items.
Guides on rinsing procedures for equipment parts before disinfection.
Describes two methods for disinfecting system components: Washer-disinfector and disinfectant fluid.
Details the procedure for steam autoclaving system components and their cycle limits.
Explains different methods for drying system components after cleaning or disinfection.
Provides instructions for reassembling system components after cleaning.
Mandates performing a system checkout after cleaning procedures.
Refers to equipment-specific user documentation for cleaning accessories.
Recommends regular inspection and calibration for system longevity.
Describes remote services for system evaluation and troubleshooting.
Explains various methods to initiate the remote service process.
Details system weights, dimensions, display features, and model variations.
Defines the critical functions that the system must maintain under all conditions.
Lists weights and maximum load capacities for system accessories and configurations.
Specifies operating and non-operating conditions for temperature, humidity, and pressure.
Lists applicable safety, EMC, and usability standards the system complies with.
Details mains power, battery specifications, and auxiliary power outlet ratings.
Specifies central gas supply pressures, connection standards, and maximum levels.
Describes gas mix, flow ranges, preset concentrations, and O2 flush specifications.
Details the AGS type, scavenging flow requirements, and outlet connection options.
Provides specifications for system volume, patient tubing, CO2 absorber, and ventilation modes.
Lists compliance, internal volume, and flow resistance for breathing circuits.
Specifies ventilator type, patient range, ventilation modes, and parameter settings.
Details parameters for administered breaths, loops, lung characteristics, and accuracy.
Specifies weight, pressure, flow, and vacuum for the O2 and suction module.
Lists weights and dimensions for miscellaneous optional equipment like vaporizer holders.
Details vaporizer agents, type, weight, dimensions, capacity, and accuracy.
Describes gas analyzer technology, measured parameters, accuracy, and response times.
Details the test method for determining gas analyzer accuracy based on respiration rate.
Lists ventilation and gas alarms with their respective limits and parameters.
Covers pollution control, hazardous substances, and compliance with environmental standards.
Specifies serial ports, USB, video out, and Ethernet communication capabilities.
Provides information about the Unique Device Identification label format and components.
Serves as information for the safe use of the device concerning electromagnetic compatibility.
Declares the device's electromagnetic emission characteristics and intended environment.
Details the system's immunity levels to electromagnetic disturbances according to IEC 60601.
Provides recommended distances for portable RF equipment to ensure compatibility.
Contains CFDA registration and product standard information for China.
Provides a template for logging system checkout procedures and results.
Provides a template for logging routine cleaning and disinfection activities.
Provides a template for logging yearly maintenance and 5000-hour service records.
| Brand | Getinge |
|---|---|
| Model | Maquet FLOW-i 4.2 |
| Category | Medical Equipment |
| Language | English |