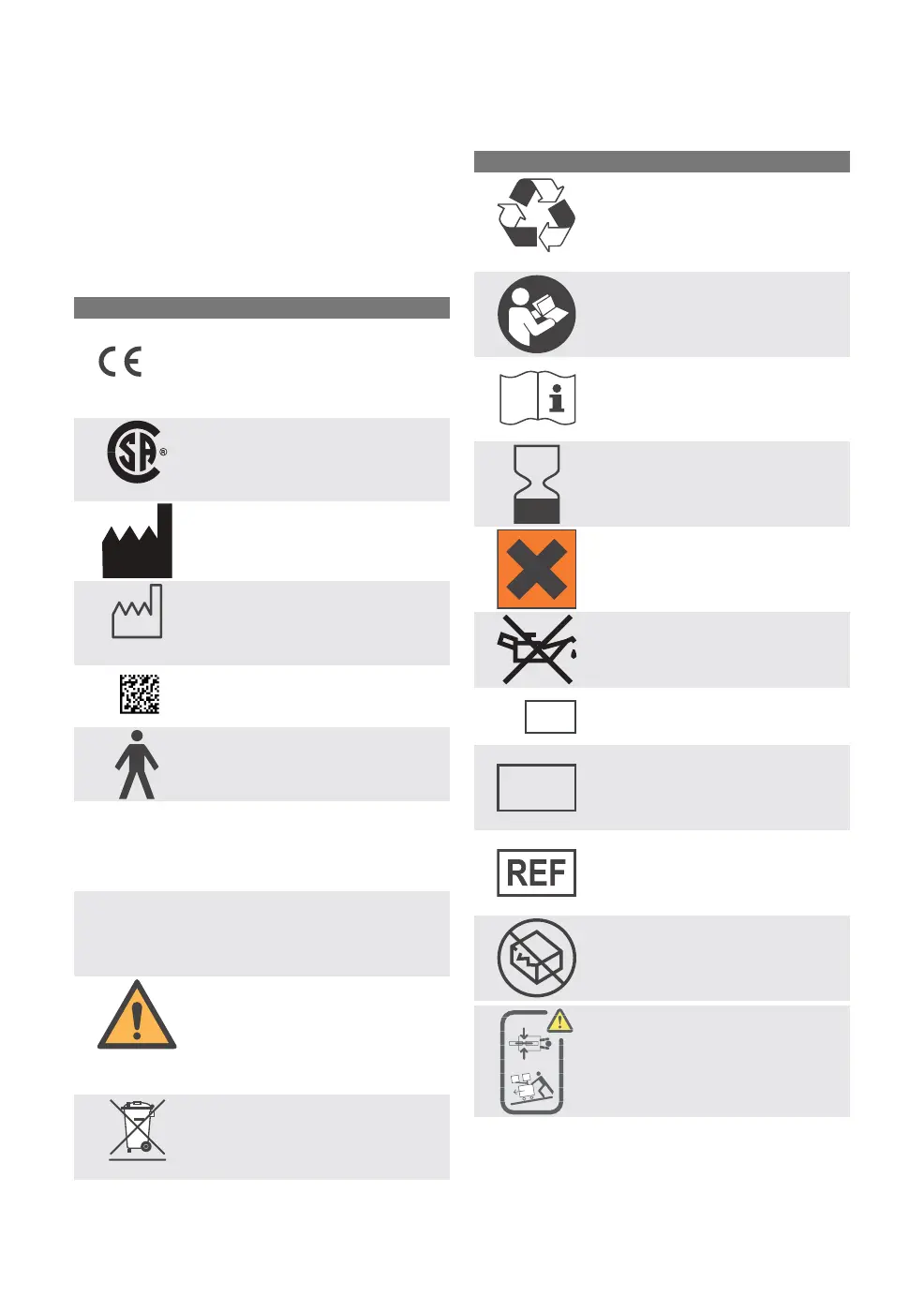
3.8 Explanation of symbols
3.8.1 Labels
The following symbols are shown on the
system:
DescriptionSymbol
CE label. The device complies
with the requirements of the
European Council Directive
93/42/EEC (i.e. the Medical
Device Directive).
0123
CSA label—Indicates compliance
with Canadian and US standards
C US
Manufacturer
Manufacturing date
2012
UDI Label - Unique Device
Identification. See technical
specifications, page 290.
Class 1 equipment, Type B. The
device classification according
to IEC 60601-1.
Federal law restricts this device
to sale by or on the order of a
physician.
Rx
ONL
IP classification: IPX1, drip proof
IPX1
Black border, black exclamation
mark over a yellow background.
Indicates critical information
about a potentially serious
outcome to the patient or the
user.
Special waste to be disposed of
in accordance with appropriate
industrial and environmental
standards.
Pb
DescriptionSymbol
Worn-out batteries must be
recycled or disposed of properly
in accordance with appropriate
industrial and environmental
standards.
White drawing on a blue
background. Consulting
accompanying documentation is
a mandatory action.
Indicates instructions that must
be followed in order to ensure
the proper operation of the
equipment.
Use by date
Black cross over an orange
background. Broken CO
2
absorber canisters may cause
skin irritation.
Use no oil. Applicable to parts
marked with this symbol.
Serial number
SN
Batch code
LOT
Article number
Do not use if packaging is
damaged
Caution must be taken when
moving the system up or down
a slope. Refer to Transport
conditions, page 38.
32
FLOW-i 4.2, User's Manual
| 3
| System overview |
 Loading...
Loading...