PROCESS THE APPROPRIATE BIOLOGICAL/CHEMICALINDICATOR WITH EVERY TRAY TO
CONFIRM STERILIZATION HAS OCCURRED. IF PROCESSING WRAPPED MATERIAL, P LAC E
THE INDICATOR INSIDE ONE OF THE WRAPPINGS.
THE CUSTOMER SHOULD USE ONLY BIOLOGICAL INDICATORS THAT HAVE BEEN CLEARED
IN THEIR MARKET. FOR U.S. CUSTOMERS, ONLY USE BIOLOGICAL INDICATORS THAT
HAVE BEEN CLEARED BY FDA FOR THE STERILIZATION PROGRAM CHOSEN
Notes for rubber and plastic tubing
– Always rinse tubing with clean water before use and do not dry them.
– Arrange the tubing on the tray so that their ends are not obstructed or crushed.
– Do not bend or wind tubes, but allow them to lie as straight as possible.
Notes for packets and packages
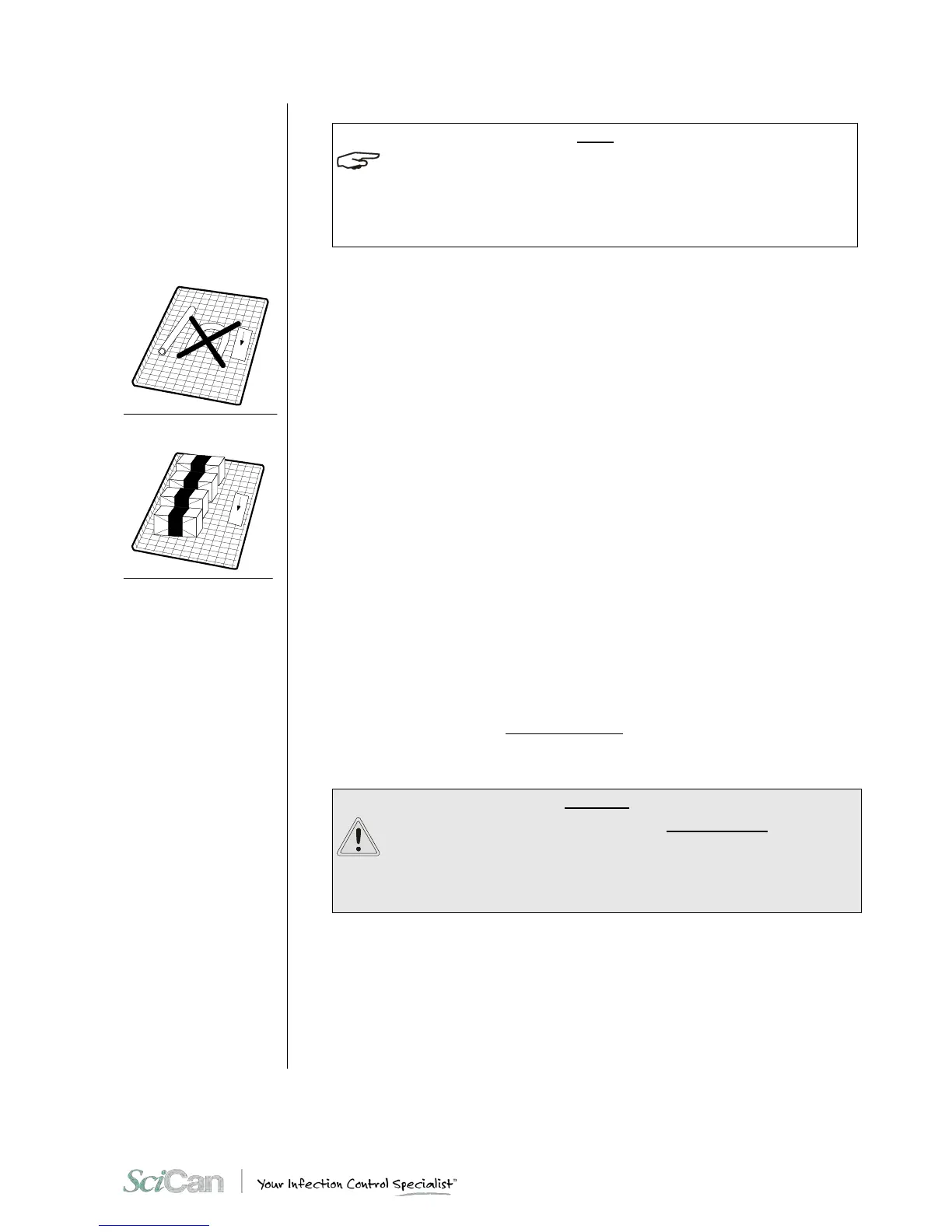
– Arrange packages side-by-side, evenly spaced and not piled, and do not allow them to
come into contact with the walls of the chamber.
– When it is necessary to wrap an object, always use suitably porous material (sterilization
paper, muslin napkins, etc.) and close the wrapping with autoclave adhesive tape.
Notes for wrapped material
– It is best to wrap instruments individually, but if more than one instrument is placed in the
same envelope, make sure that they are made of the same metal;
– Seal the wrapping with adhesive tape designed for autoclaves or heat-sealing machines.
– Do not use staples, pins or other fasteners since they can compromise the maintenance of
sterility.
– Arrange the envelopes to avoid forming air pockets that obstruct the correct penetration
and removal of the steam.
– Orient the envelopes with the plastic side up and the paper side down.
– Always check that envelopes are correctly positioned and turn them over if necessary.
– If possible, place the envelopes on their sides using a suitable support.
– If pouched or wrapped loads are not dry when they are removed from the chamber, the
instruments must be used immediately or resterilized.
IF YOU EXPECT TO STORE INSTRUMENTS, AL W AY S WR AP THEM. SEE
THE CHAPTER 10 – STORING STERILIZED MAT E RI AL .
THE USER SHOULD USE ONLY STERILIZATION WRAPS THAT HAVE
BEEN CLEARED FOR THEIR MARKET. FOR U.S. CUSTOMERS, ONLY
USE STERILIZATION WRAPS THAT HAVE BEEN CL E AR E D BY FDA FOR
THE STERILIZATION PROGRAM CHOSEN.
STERILIZATION
MONITORING
Chemical process monitors suitable for steam sterilizers at the indicated cycle temperatures
and times should be included in or on each package or load being sterilized. In addition,
SciCan recommends the use of biological monitors such as the EZTEST-STEAM indicator or
the 3M Attest system for routine monitoring of the sterilizer. It is important to select the correct
biological indicator for the cycle being tested.
 Loading...
Loading...