11.2.1.3 X-300M FDA and IEC approvals
The following devices meet the FDA and IEC requirements listed below:
Product line Product
group
Device:
SCALANCE
(Variant) Fulfills FDA and IEC require‐
ments
X-300 X-300M X308-2M - -
X-300 X-300M X308-2M TS - -
Note: In the modular devices (M), the marking is provided by the MM900 media modules and the SFP transceivers.
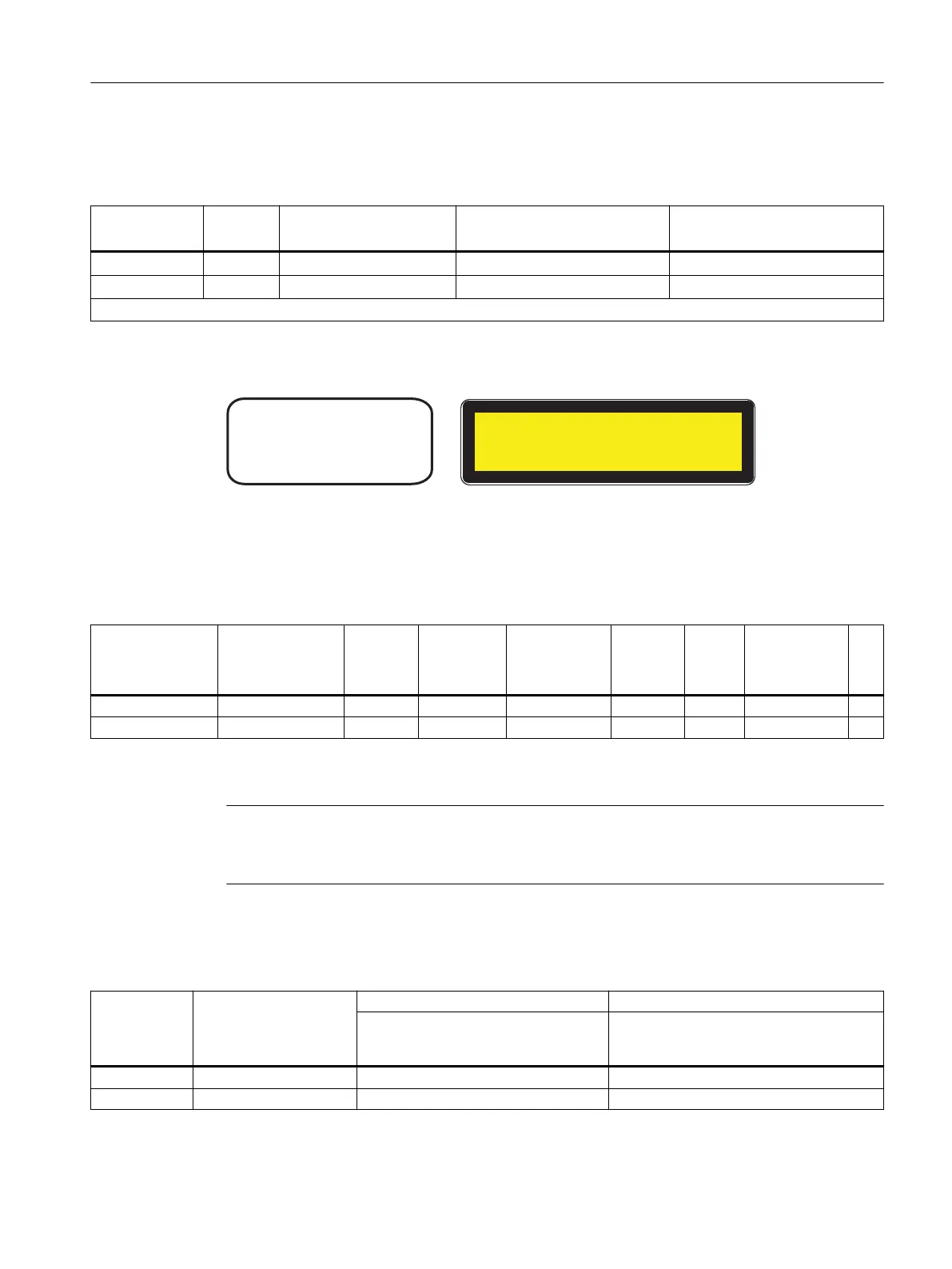
&RPSOLHVZLWK&)5
DQG
&/$66/$6(5352'8&7
)'$
,(&
Figure 11-2 FDA and IEC approvals
11.2.1.4 Overview of X-300M approvals
Table 11-2 Overview of the approvals
Device:
SCALANCE
(Variant) c‑UL‑us c‑UL‑us
for hazard‐
ous loca‐
tions
1
FM
1
C‑TICK CE ATEX95
Zone 2
1
E1
X308-2M (-) ● ● ● ● ● ● -
X308-2M TS (-) ● ● ● ● ● ● -
1
For temperature information "T.." or the maximum ambient temperature "Ta:..", refer to the
type plate.
Note
Shipbuilding approval
No applications for shipbuilding approvals will be made for the SCALANCE X-300M.
11.2.1.5 X-300M mechanical stability (in operation)
Device:
SCALANCE
(Variant) IEC 60068-2-6 vibration IEC 60068-2-27 shock
5 – 9 Hz: 3.5 mm
9 – 150 Hz: 1 g
1 octave/min, 20 sweeps
15 g, 11 ms duration
6 shocks per axis
X308-2M (-) ● ●
X308-2M TS (-) ● ●
Certifications and approvals
11.2 Product group X-300M
SCALANCE X-300
Operating Instructions, 11/2019, A5E01113043-24 261

 Loading...
Loading...











