The ACD measurement is the distance between corneal epithelium and the crystalline lens surface.
The light emitted from this instrument is NOT hazardous. It is classified ad Group 1 of ISO 15004-2.
15.4 Performance Testing
BRIEF SUMMARY OF PERFORMANCE TESTS AND RESULTS
A prospective, single site clinical study comparing the performance of the ALADDIN with IOL Master was
conducted in 63 eyes (1 eye for each enrolled subject). This study evaluated the agreement, repeatability and
precision in the subsequent endpoints:
Anterior Chamber Depth
Radii of curvature of flattest and steepest meridian
Axis of the flat meridian
White to white distance
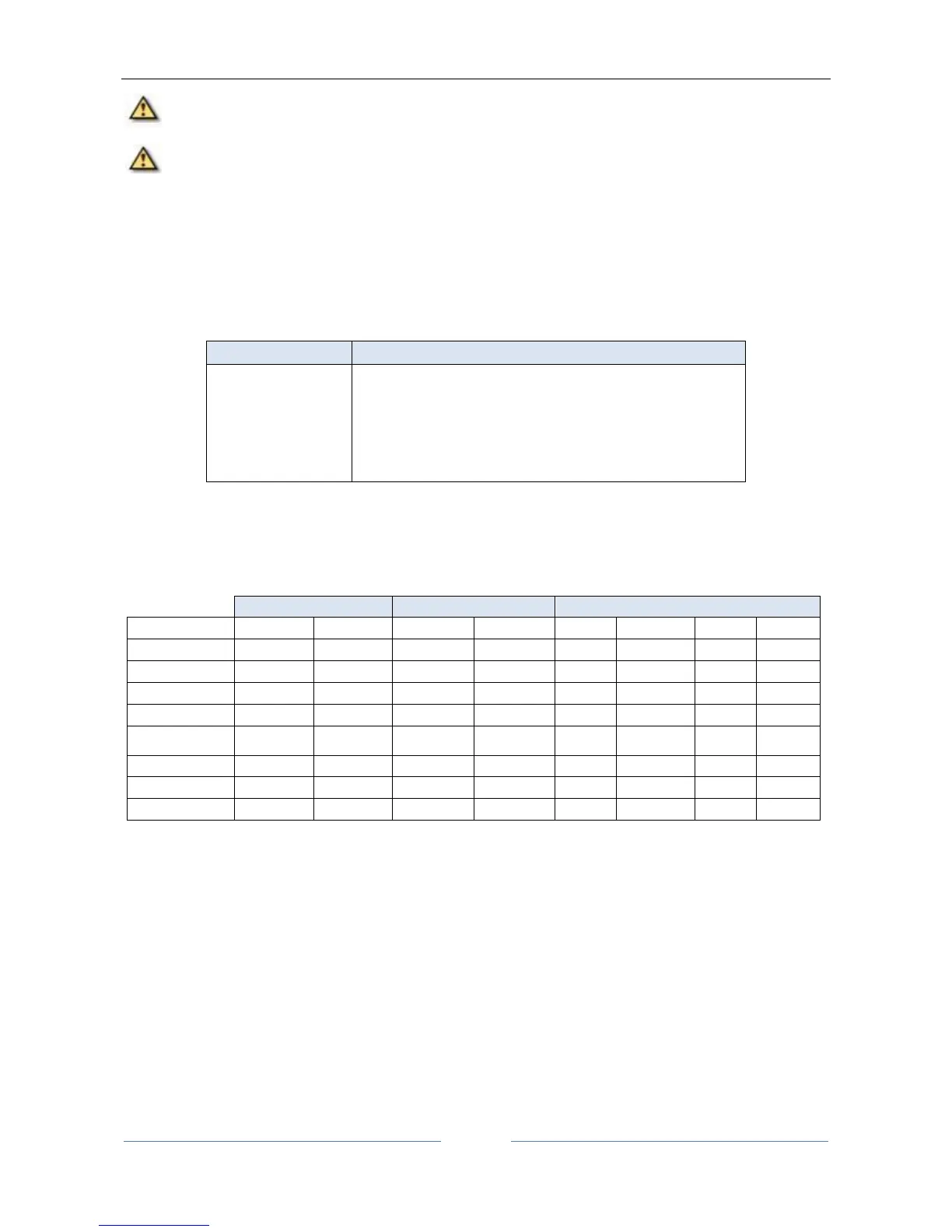
The agreement between instruments is summarized in Table 1:
Table 1.
Agreement between ALADDIN and IOL Master.
Note to the Table 1:
- axis results are summarized by the subsequent classes of cylinder: 0.00cylinder0.75; 0.75<cylinder1.50; 1.50<cylinder.
- data in table concern the comparison producing the worst mean difference for al detected in the study.
The results of the study demonstrate that the axial length, corneal radius, anterior chamber depth and the
“white-to-white” measurements of the Aladdin are substantially equivalent to those of the predicate
device.
To evaluate repeatability and reproducibility, for each endpoint (AL, ACD, AX, K1, K2, WTW), analysis of
precision was performed for ALADDIN and IOLMaster separately using a factorial ANOVA models for repeated
measures within subject including the subsequent term: device identifier (A1, A2, A3 for ALADDIN and I1, I2,
I3 for IOLMaster), operator identifier (1, 2 and 3), subject identifier (1 to 63) and every two-way interactions;
a standard variance component matrix was used as covariance structure of R-side matrix. The related results
 Loading...
Loading...