Meter Setup
114
In some circumstances, it may be useful for operators to confirm the validity of their results. To do
so, an option can be enabled that prompts operators to confirm the results of every test.
9 Touch Result Confirmation.
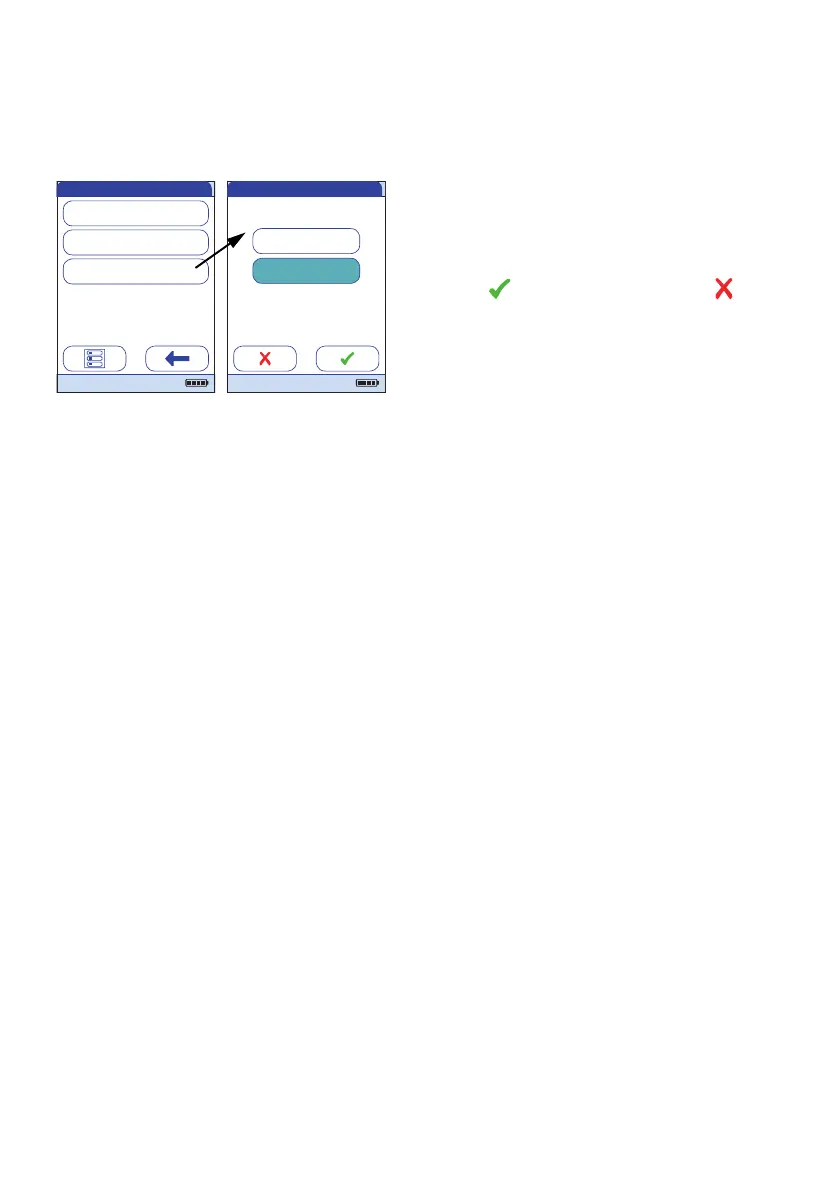
10 Touch the button to select the state of the
option. Your selection is now highlighted.
11 Touch to save this setting, or touch
to exit this menu without saving any
changes.
Setup - Optional Screens
Result Login
Result Confi rmation
Start Info
04/19/2016
Disable
Enable
Res. Confi rmation Screen
04/19/2016
 Loading...
Loading...